Here’s a popular high school chemistry fact: Helium atoms don’t interact with other atoms to create compounds. Well, that fact might need some reevaluating.
The surface of Jupiter, a planet which might have helium compounds
An international team of scientists think they have created a stable helium compound, meaning one composed of both helium and sodium atoms together. The discovery would be wild not only because of the way it goes against some of our basic assumptions of chemistry, but it would also help scientists better understand the way atoms act in the high-pressure centres of gas giant planets.
“Chemistry changes when you apply high pressure, and this can be achieved inside our Earth and on different planets like Saturn,” study co-author Ivan Popov, a doctoral student at the University of Utah, told Gizmodo. “But this,” chemistry involving helium, “is a book changer.” Other noble gasses, like xenon and argon, have previously been shown to bond with magnesium under high pressures.
So, chemistry lesson: Atoms are positively charged nuclei surrounded by negatively charged electrons. The more positive protons in the nucleus, the more electrons the atom can hold onto. The electrons are sorted into layers, called shells. Atoms bond by sharing electrons in their outermost shells, but if that shell is full, they normally won’t bond. Noble gasses like helium or neon are atoms with full outer shells, so bonding with them is like trying to hold hands with someone already toting a pair of bowling balls. That’s why the new study’s result is so interesting, if it holds up.
“Ad hoc, it sounds pretty wild,” Reinhard Boehler, a research scientist at the Carnegie Institute for Science in Washington, DC told me from his car when I described the study’s results to him.
In their study, the researchers loaded helium gas and sodium crystals into a cavity between a pair of diamonds, and squeezed the atoms at super-high pressures. The resulting compound was a solid arrangement of alternating sodium and helium atoms in a cube shape, with electrons shared between them. “It’s not a real bond” in the sense of the ionic and covalent bonds you learned about in chemistry, explained Popov. “But [the helium] does stabilise the structure. If you take those helium atoms away, the structure will not be stable.”
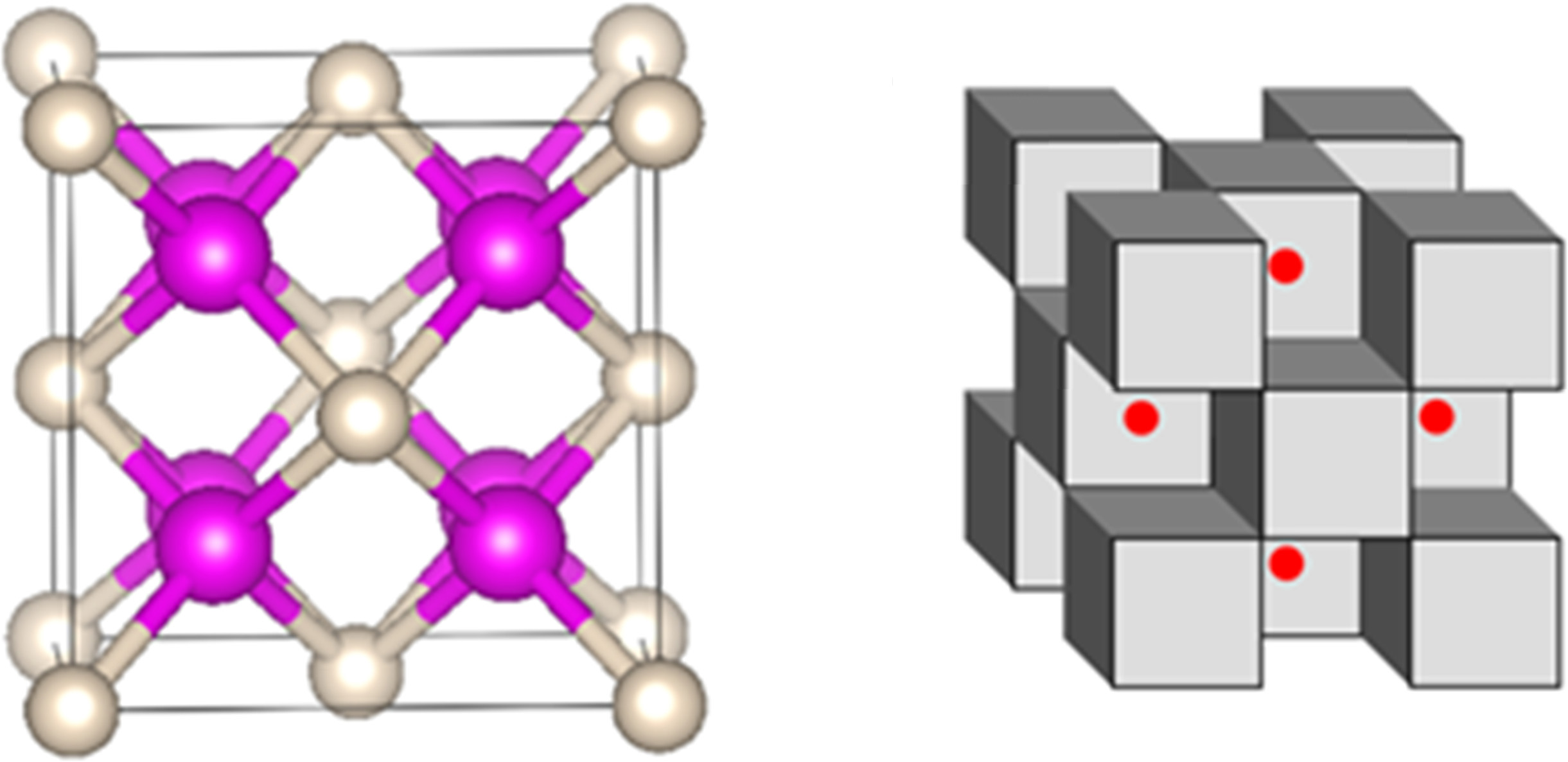
On the left, the pink atoms are sodium and the white are helium. On the right, the cubes represent helium and sodium while the red dots represent the shared electrons. Image: Ivan Popov/Utah State University
If this sounds familiar, it may be because last week, researchers announced they’d produced metallic hydrogen using a very similar high-pressure experimental setup — though other scientists had a lot of doubts about that result. This time around, at least one scientist found the results more convincing. “This is much sounder science,” Henry Rzepa, a professor at Imperial College, London, who was initially sceptical when I told him about the new study, said. “This helium compound is a breakthrough.” He pointed out that, of course, other scientists would need to complete similar experiments in order to verify whether a helium compound had actually been found.
Rzepa further noted that this compound isn’t your standard mixture of bonded elements you learned about in chemistry class, but an “electride”. Such molecules consist of electrons dispersed within a crystal structure, but the structure itself is somewhat mysterious to us. “We have long suspected that a quite different chemistry, controlled by rather different if not very different rules, must exist under extreme conditions such as ultra high pressures,” he said. “This [sodium-helium compound] offers a tantalising glimpse into this new frontier of chemistry.”
That means these compounds won’t have much use on Earth, and can only exist under strange conditions like those at the centre of gas giant planets like Jupiter and Saturn. The helium-sodium compound could therefore give us insight into what chemistry might be like inside other planets, the paper’s authors write, since those gas giants have plenty of helium.
So, at least one pair of eyes thought this wild compound really could exist.
