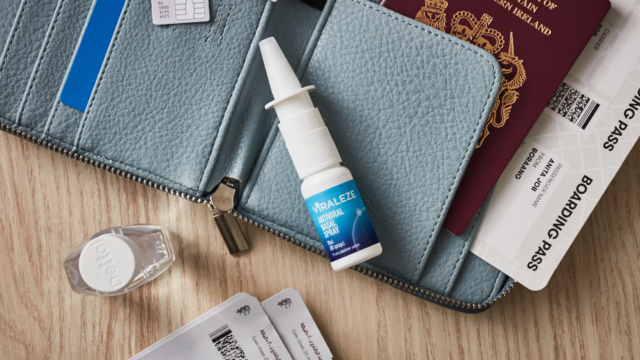
Melbourne-based biotech company, Starpharma, has been slapped with a $93,000 fine for breaching advertising regulations for its new nasal spray, which allegedly had COVID-fighting properties.
The Therapeutic Goods Administration handed down seven infringement notices to Starpharma, totalling $93,200 worth of fines in relation to its Viraleze nasal spray.
“The alleged advertising, on two of Starpharma’s websites, included a restricted representation claiming that Viraleze is an antiviral nasal spray that stops SARS-CoV-2, the virus that causes COVID-19. Any claims or references to preventing or treating a serious form of a disease, condition, ailment or defect are restricted representations,” the TGA said in a statement.
New data shows VIRALEZE™ active SPL7013 has potent and rapid virucidal activity, achieving >99.9% reduction of virus against the Alpha (UK), Beta (South Africa) & Gamma (Japan/Brazil) SARS-CoV-2 coronavirus ‘Variants of Concern’, in laboratory assays. https://t.co/uA8uH3a6vu pic.twitter.com/B5azHG0u5F
— Starpharma (@Starpharma_ASX) June 18, 2021
A major concern raised by the TGA is the fact that the product contains the active ingredient astodrimer sodium, which is classified as a pharmacist-only medicine in Australia.
[related_content first=”1709012″]
Astodrimer sodium “is not permitted in advertising to consumers and was not authorised or required by a government or government authority” for coronavirus-related treatments.
At the time of publishing, Viraleze is not authorised for use or sale in Australia, yet Starpharma reportedly promoted the product – and its COVID-fighting properties – on its website and YouTube channel.
According to the TGA, Starpharma’s advertising “included a restricted representation claiming that Viraleze is an antiviral nasal spray that stops SARS-CoV-2, the virus that causes COVID-19. Any claims or references to preventing or treating a serious form of a disease, condition, ailment or defect are restricted representations.”
The product has never been available in Australia, despite advertising material being viewable to Australian shoppers until very recently.
However, the sale of Viraleze in the UK was temporarily halted last month after Starpharma’s retail partner LloydsPharmacy was hit with an investigation by the UK’s equivalent of the TGA.
Despite the company not agreeing with the concerns raised by the regulator, Starpharma agreed to pause product sales until the situation can be resolved.
Following the TGA’s findings, Starpharma – which is an Australian Stock Exchange-listed company specialising in sexual health products – released a statement to the ASX regarding the matter.
“The company will work closely with the TGA to resolve the current matter and how to balance the need to provide information to its shareholders about key company milestones…with requirements of the [Therapeutic Goods] Act in relation to advertising in Australia,” Starpharma said in a statement on Monday.