A group of scientists say they’re on the verge of developing a promising treatment for chronic pain that works by turning down, but not permanently altering, a gene that helps us sense pain. Their new research with mice suggests that the gene therapy could offer months of pain relief at a time without any major health risks. Still, more work has to be done before we could see trials in people.
Chronic pain remains one of the most common and challenging ailments to treat. Estimates vary, but a 2018 study found that 20% of American adults experience chronic pain, while 8% had pain serious enough to highly impact their daily functioning. Though there are many painkilling treatments available, most only provide mild and/or short-lasting relief. More powerful drugs like certain opioids can offer significant pain relief, but not everyone responds well to them, and they carry a risk of addiction and other serious adverse effects. Even when opioids do work as intended, the body gradually becomes used to them, and they can lose potency over time.
Researchers at the University of California, San Diego had been looking into the possible applications of genetic engineering when one of them, Ana Moreno, came across an intriguing paper, involving a gene called SCN9A. The gene is commonly expressed (meaning that it’s actively working) in nerve cells outside of the brain and spinal cord, also called the peripheral nervous system. The paper detailed how mutations that made the SCN9A gene nonfunctional also left people with the inability to register and feel pain. Other research has shown that mutations that increased the expression of SCN9A in these cells left people with a heightened sensitivity to pain.
SCN9A controls a piece of our cells’ machinery called a sodium channel, specifically a subtype known as Nav1.7. In the nerve cells where it’s found, Nav1.7 seems to be crucial to their ability to recognise possible pain signals that are then sent to the brain, which made Moreno and her colleagues curious. If they could find a way to safely dampen Nav1.7 through gene therapy, they reasoned, they should be able to help people with chronic pain. And because the gene has a simple function that seems to only affect Nav1.7, any therapy would likely be low in risk to people.
“What’s very exciting about this approach is that it targets a gene that has a very clean phenotype,” Moreno, a recent PhD graduate in bioengineering and biomedical engineering from UCSD, said in an email. “As we know that this gene is directly involved in pain, it’s a perfect target that is non-addictive to allow us to provide relief for chronic pain patients.”
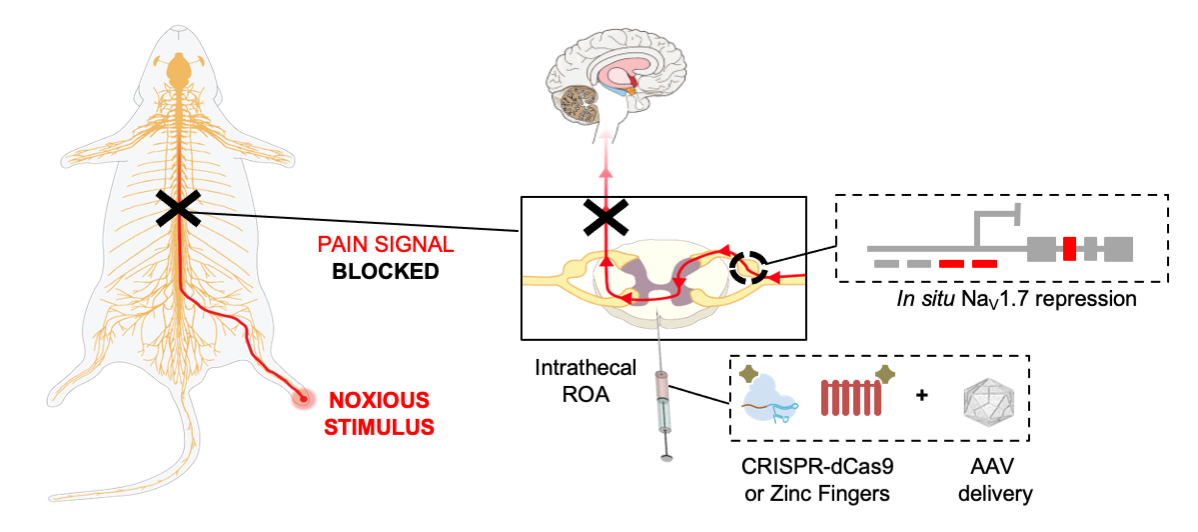
Their new work, published in Science Translational Medicine on Wednesday, tested out two common methods of genetic engineering on mice, the more recently developed CRISPR/Cas-9 and an older technique that relies on zinc-finger proteins. Though CRISPR is more commonly known for permanently editing genes, the team decided to use a modified version of each method (delivered to cells through a neutered virus) that would only block the expression of SCN9A/Nav1.7 temporarily, not turn it off forever.
The mice they used were meant to develop different types of chronic pain, such as inflammatory pain commonly linked to chemotherapy or nerve pain. To measure their response to pain, the researchers looked at, among other things, how long the mice could tolerate stimuli like heat and touch. Across their experiments, the mice given the gene therapy appeared to have a higher tolerance to pain and experienced long-lasting pain relief in general (likely the equivalent of weeks to months in people). Also importantly, the treatment seemed to have no noticeable adverse effects on the mice.
“Our studies demonstrated efficacy, safety, and longevity,” Moreno said. “This has a lot of potential to help patients in the clinic that have intractable pain.”
Of course, these results are only the beginning of something still largely untested. Mice are not people, and there are possibly important differences in how their version of Nav1.7 works from our version. While the team’s treatment appears temporary and safe on paper, they can’t rule out any harmful unintended effects from it just yet. Moreno noted that in people unable to feel pain due to their dysfunctional SCN9A gene, they also experience ansomia, or an inability to smell. In mice, the team didn’t find any reduction in smell perception, but it’s one risk among others that they’ll have to keep an eye out for.
“As we move forward, we will need to perform robust toxicity studies to determine the safety profile in larger animal models,” she said.
To help make their experimental treatment a reality, Moreno has since founded the company Navega, along with her co-author Prashant Mali, who she studied under during her time at UCSD (co-author Tony Yaksh, a UCSD anesthesiologist and pain researcher, is also a scientific advisor to Navega). The researchers next plan to tweak their method for use in humans, while moving onto trials with non-human primates. Should things continue to go well, it may not be too long before they can start to test it out on people.
“We believe we will reach clinical trials in two years,” Moreno said.
