Today we got the first substantial look at data on one of the covid-19 vaccines likely to reach the U.S. general public later this month. The Food and Drug Administration and the companies Pfizer and BioNTech released summary documentation of the trial data collected so far on Pfizer and BioNTech’s vaccine candidate. The lengthy materials, among other things, offer a preview of what it might feel like to get the two shots that make up the vaccine.
The documents have been released to the public in the lead-up to a pivotal meeting happening this Thursday, when an advisory committee of outside experts will evaluate and discuss the trial data before making a recommendation on whether the Pfizer/BioNTech vaccine should be granted an emergency use authorization by the FDA. The companies’ documents summarize what the two companies plan to present to the committee, while the FDA’s documentation is the agency’s review of the data. Together, the documents represent the first real analysis of Pfizer/BioNTech’s results outside a corporate press release.
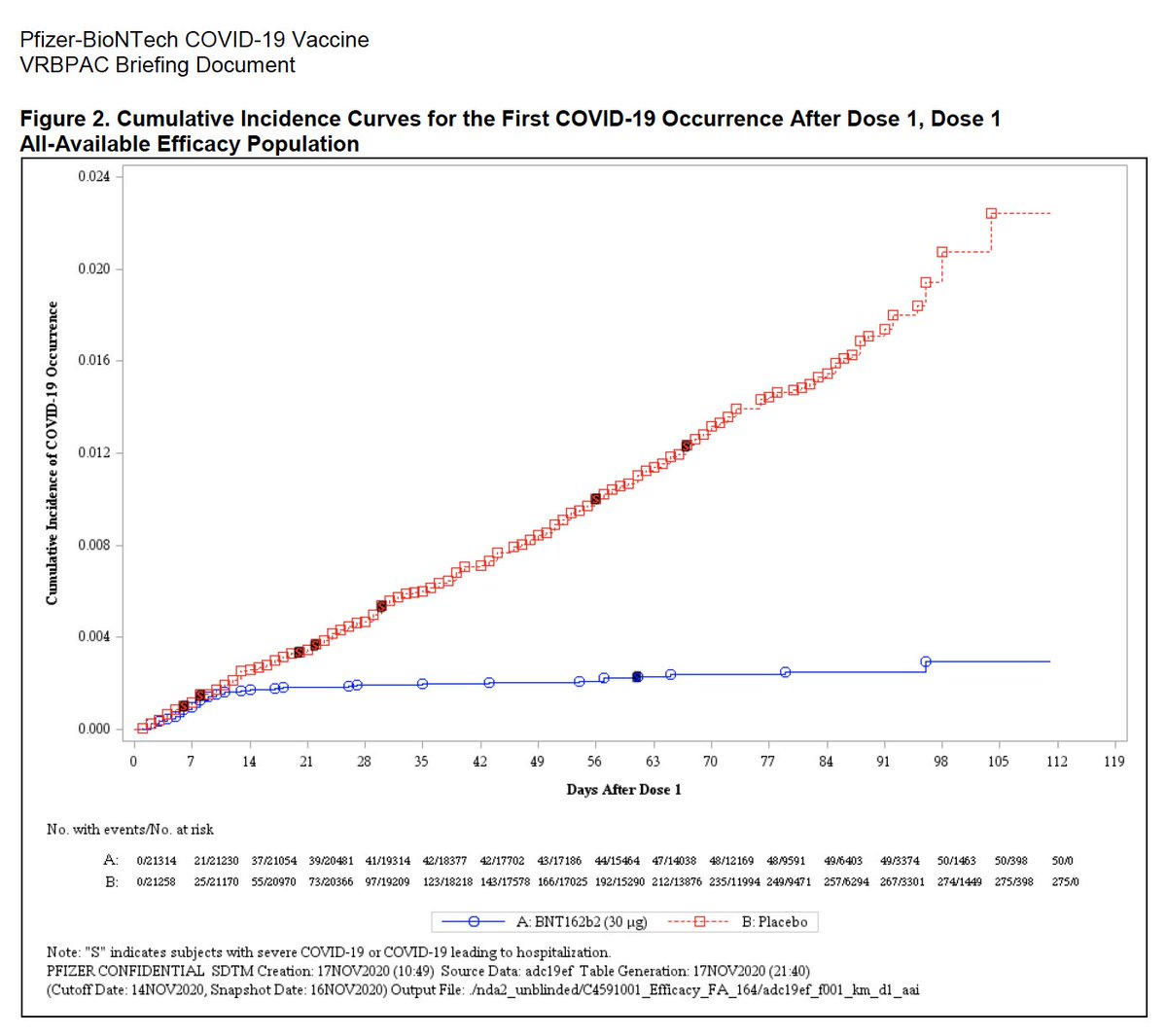
There’s a lot of encouraging information to be found here. Data from the Phase III trials conducted so far, for instance, suggest that the first dose of the vaccine can provide a substantial amount of immunity within two to three weeks. One especially illustrative graph (seen left) shows that the risk of symptomatic infection drops significantly in the vaccine-treated group compared to the placebo control group after one dose. Of course, it’s only after the second dose, taken a month later, that the vaccine appears to reach the roughly 95% effectiveness seen in the overall data.
Perhaps just as importantly, the safety data looks reassuring. When scientists or health agencies want to evaluate how safe a new treatment or vaccine seems to be in a clinical trial, they look for adverse events, which are simply medical problems that happen to someone during the trial (the problem may or may not have been caused by the drug being studied). If any one adverse event is more common in the treatment group than the control group, it’s more likely to be a true side effect of the treatment.
According to the FDA’s review, there were no severe or life-threatening adverse events linked to taking the vaccine among 38,000 participants and no “specific safety concerns identified that would preclude issuance of an EUA.”
That said, no drug comes without side effects. Hopefully, the more we know about what to expect going in, the less likely we’ll be freaked out by feeling sick after the shot — or to misattribute the side effects for the actual illness, as often happens when people wrongly swear that they got the flu from the flu shot.
The safety data comes from 38,000 volunteers who were given two doses and were tracked for at least two months (another analysis done with an extra 5,000 volunteers later in the trial found no significant differences).
Here’s the breakdown:
-
84.1% had injection site reactions like pain or itching in the seven days following the first and/or second dose
-
62.9% experienced fatigue
-
55.1% experienced headache
-
38.3% experienced muscle pain
-
31.9% had chills
-
23.6% had joint pain
-
14.2% had fever
In other words, a lot of people experienced what might be called flu-like symptoms.
These symptoms usually lasted a few days at most, and fewer than 5% of volunteers experienced any adverse reaction considered severe (severe here meaning intensity, like a really throbbing headache, not serious in the sense of being life-threatening). Interestingly enough, younger people were more likely to experience a severe adverse reaction than people over the age of 55. But otherwise, there weren’t any noticeable differences in the safety profile of the vaccine across age, gender, or race and ethnicity. People as young as 16 to 17 — still considered children — did take the vaccine as well, and the limited data so far suggests that it’s just as safe for them as it was for adults.
There are still longer-term questions that will take time to figure out for any vaccine that reaches the public. It’s not yet clear whether Pfizer/BioNTech candidate or the similar Moderna vaccine are effective at preventing transmission of the coronavirus altogether, or if vaccinated people can still carry an infection silently if exposed and transmit it to other, unvaccinated people. That’s important to know, because asymptomatic transmission could then still be a real risk for the foreseeable future, at least until enough of the population is vaccinated. We also don’t know how long this vaccine-provided immunity will last, though there’s reason to be optimistic there.
There are other gaps in the data. For instance, we don’t know how pregnant women would respond to the vaccine, because they weren’t studied at all (a long-standing problem in clinical trial research). So they won’t have access to any vaccine initially, if ever. Younger children are now being studied in vaccine trials, including Pfizer/BioNTech’s, but it will take time for the safety data to emerge, so they’ll also be later on the list of who gets a vaccine.
One interesting footnote concerns people who had already contracted covid-19 but received the vaccine anyway. According to the FDA, a secondary analysis suggests the vaccine has benefit in preventing covid-19 among individuals previously infected with the coronavirus, “although available data for these outcomes did not allow for firm conclusions.” We know reinfection can occur, though it appears to be rare.
All in all, it looks like good news. And we’ll almost certainly know whether the first covid-19 vaccine will be approved in the U.S. by week’s end.