The extremophile bacterium Deinococcus radiodurans was first discovered in 1956 at Oregon State University, where it was busy ruining a gamma ray experiment designed to sterilise a tin of ground meat. The “sterilised” meat spoiled, thanks to D. radiodurans and its preternatural durability in the face of radiation: The hearty microbe can withstand somewhere up to 5,000 grey (Gy), or about 1,500 times the dose that would kill a human being.
D. radiodurans is unusual even in the world of extremophiles, in that it has evolved not to thrive in one uniquely difficult environment but in many. (The extremophile microbe Pyrolobus fumarii, to cite a more common example, is found almost exclusively near hydrothermal vents in the deep sea.) Scientists have called D. radiodurans “a robust ‘generalist’” capable of persevering amid prolonged exposure to everything from toxic chemicals and corrosive acids to desiccating desert heats and subzero temperatures. In the decades since decomposing that meat, colonies of the bacteria have been found occupying the coolant water tanks of nuclear reactors and thriving improbably on the weathered granite of Antarctica’s dry valleys. They’ve faced exposure to solar radiation and the vacuum of space onboard a European Space Agency satellite and have survived punishing simulations of life on Mars at the German Aerospace Centre in Cologne.
How D. radiodurans manages to do all this is still a contentious topic among the small international community of researchers who study it — but few have devoted as many decades to answering this question as Mike Daly, a molecular biologist at Uniformed Services University, a medical college run by the Pentagon in Bethesda, Maryland.
“One of the reasons perhaps that so few people are working on it is because many of the mysteries have been solved,” Daly told Gizmodo. He estimates that there are “maybe 10” labs in the whole world fully dedicated to studying D. radiodurans. “The great questions that we had 20 years ago about what makes this thing so radiation resistant — they have been solved completely in the sense that we have now built on those insights.”
Over the years, both NASA and the National Academy of Sciences have sought out Daly’s advice on purging D. radiodurans from space probes in order to avoid contaminating other worlds. He has prepared an engineered strain of D. radiodurans for work cleaning up radioactive waste sites, reducing toxic hexavalent chromium to nontoxic chromium and decomposing toluene solvent pollutants into safer substances at the sprawling Hanford nuclear production complex in Washington state. In recent years, Daly has been investigating ways to apply the lessons learned from this bacteria’s unique radiation resistance to the production of faster, cheaper, and safer vaccines.
Earlier this year, Daly and his group at USU published the results of their efforts to produce an inactivated polio vaccine, a project they undertook with the biotechnology firm Biological Mimetics, founded by several scientists formerly with the National Cancer Institute. “A lot is known about polio,” says BMI president Greg Tobin, a virologist who had previously led SAIC-Frederick’s Gene Expression Regulation Group at the institute. “So, if you wanted to pick something to characterise immunity, polio is a pretty good ‘proof of concept.’”
At the core of Daly’s new vaccine production method is a crucial (but not especially intuitive) mechanism by which D. radiodurans protects itself from cosmic rays and other forms of ionizing radiation. For decades, the prevailing assumption among radiobiologists had been that the microbe’s key strategy for withstanding radiation damage involved active cellular mechanisms for directly protecting its DNA, including a set of novel DNA and RNA repair proteins produced in response to radiation exposure. But, as Daly and his colleagues began noticing in the early aughts, D. radiodurans has a more indirect approach: It focused primarily on insulating all of these repair proteins, protecting this relief crew from harm as they ventured out to clean up and rebuild the genome.
You can think about what harsh radiation does inside a cell like it’s the microscopic equivalent of boiling a pot of pasta until all the noodles dissolve into incoherent, mushy blobs. Both gamma rays and x-rays energize and break apart water molecules inside a cell during radioactive bombardment, generating highly reactive “oxidizing” compounds from all the newly freed-up hydrogen and oxygen ions. More so than the radiation itself, it’s those oxidizing compounds that wreak havoc across all the little organelles and useful macromolecules inside the cell. To combat this and save D. radiodurans’ repair proteins, each bacterium manufactures a special antioxidant compound containing positively charged manganese. (Some of the human body’s own critical antioxidants, like the enzyme superoxide dismutase, also employ this same mineral element. Manganese alone outside of these compounds is a very powerful antioxidant.)
“We’ve shown that these manganese complexes are phenomenally good at protecting proteins from oxidants generated during radiation,” Daly said. “But, these same manganese antioxidants, they did not protect DNA or RNA. So, as soon as that became very obvious, I said to myself, ‘It sounds like an ideal way of making a vaccine.’”
Any good vaccine needs to resemble the infectious microbe that it is training the body’s immune system to search and destroy, without being as harmful as the real thing. In the past, this has been a fraught balancing act. In 2012, for example, the Centres for Disease Control and Prevention documented somewhere near 42,000 new cases of whooping cough, the largest outbreak since 1955, due not to poor access to healthcare, or kooky anti-vaxxers, or a heartier strain of the pertussis bacteria itself, but to an unreliable vaccine composed of fragmentary cellular material.
“If you can grow-up your pathogen (whatever it is), if you mix it in with these manganese antioxidants,” Daly said, “you should be able to obliterate the genome, whether it’s RNA or DNA, and render it completely non-infective and sterile, while at the same time preserving all the structures and peptides, all the ligands and all the things that make up the surface of the virus or bacteria. Then you’ve sort of got like a ghost of what the real thing is.”
Today, the reigning speed record for vaccine development is still Jonas Salk’s vaccine for polio: a six-year undertaking split between about four years’ worth of basic research (the arduous process that Daly’s method could dramatically cut down) and two years’ worth of clinical trials (which it could not).
Complex “trial and error” experimentation is baked in to many of both the traditional and more modern methods of vaccine development. While offering efficiency and cost savings, contemporary subunit vaccines — which use recombinant DNA to produce only those portions of a virus that are capable of inducing a safe immune response — each require their own indefinite period of lab research to determine which parts of the pathogen actually will work. More old-school methods, like producing an inactivated whole virus vaccine with some combination of heat and chemical treatments, skip most of that time-consuming investigative effort, but often damage enough key surface proteins in the process that the immune response can be pretty weak. (This weakness, incidentally, is where the need for booster shots comes from.)
Across the gamut of theoretically promising vaccine development concepts, a lot of laborious tinkering tends to crop up in practice: Strains of a virus need to be identified and compared; genomes need to be mapped to find the code for those most useful surface proteins; inactivation methods need to be tweaked, adjusted, or wholly rethought for a given virus. Essentially, what Daly’s team has done is figure out a way to sidestep most of this to make a classic inactivated whole virus vaccine, very quickly and with negligible damage to the critical antigenic proteins on the bug’s surface.
When asked to independently review some of Daly’s published research for this article, Thanh Nguyen, a mechanical engineer at the University of Connecticut whose work focuses on tiny biodegradable structures for vaccine delivery, described this manganese complex method as “really exciting” and “definitely significant.”
Similarly, infectious disease doctor Sandip Datta — currently a clinical director at pharmaceutical giant Merck and previously head of the National Institutes of Health’s Bacterial Pathogenesis Unit — agreed with Daly, his former vaccine research collaborator, that this production method could be uniquely suited for expediting vaccine candidates during a pandemic, like the current covid-19 crisis.
But Jean Peccoud, a molecular biologist at Colorado State who recently wrote about the competing approaches to a coronavirus vaccine for The Conversation, worries that Daly’s manganese complex method may hit complications during any attempt at mass production. While labs across the world have proven capable of growing the coronavirus in a cell culture — a highly scalable approach compared to, say, the time-consuming method by which flu shots have been incubated in hen’s eggs for the past 70 years — there are still obvious potential risks to growing up industrial quantities of a lethal pathogen.
“If you look at who is winning the race to develop a vaccine,” Peccoud told Gizmodo, “none of the leading teams are approaching it by growing the virus on a large scale.”
Of the approximately 115 different coronavirus vaccine candidates currently being fast-tracked, the furthest along have involved a method borrowed from gene replacement therapy, whereby a harmless adenovirus is created with the genetic material required to produce some (but not all) of the coronavirus’ telltale surface proteins. Oxford University’s Jenner Institute and its industry partners, as well as the Chinese biotech firm CanSino and China’s Academy of Military Medical Sciences, have reported success testing their adenoviral vector vaccines. But as The New York Times pointed out in its recent coverage, “Neither technology has ever produced a licensed drug or been manufactured at scale.”
Peccoud suspects that “this technology with the manganese complexes might be more relevant if coronavirus stays around for years and we need to have a really good vaccine because it’s becoming an endemic disease,” like malaria in tropical regions or chicken pox among school children across the world. “Maybe the vector vaccines, because they are going after a single antigen, their efficacy is not going to be as high as a whole virus vaccine.”
For Tobin’s group at BMI, the way Daly’s approach innovates upon this very old and well-established method for vaccine development (the inactivated whole virus vaccine) is in fact its strong suit. “These ‘conventional’ approaches might not seem as modern,” Tobin said, “but they are backed with decades of successful experience and would be a much more certain investment in time and resources.”
Some of the most sceptical perspectives on Daly’s manganese complex vaccine concept have come from his old peers in radiobiology, who have also studied D. radiodurans but have tended to focus on the bacterium’s genetic expression of arguably unique or exceptional DNA- and RNA-repair proteins. While some of them have rejected Daly’s view that these manganese antioxidants evince a full-on paradigm shift in our understanding of how D. radiodurans and other organisms resist radiation, they have since come around to the idea that the presence of these compounds does at least play a critical role in the process.
“There was some strong pushback, and I think there still is,” said Michael Cox, a past D. radiodurans researcher, recalling the reaction among microbiologists and radiobiologists when Daly’s team first proposed that “very high intracellular manganese” was the secret to the bacteria’s resolute radiation defence in the November 5, 2004 issue of Science. “I think it’s going to turn out that Daly was probably right, and I think it’s going to turn out that there were other factors involved, as well.”
Chemist John Battista, whose lab at Louisiana State University in Baton Rouge has used DNA microarrays to identify which parts of the D. radiodurans genome are responsible for expressing its novel repair proteins, also suspects that, “in the end, it will be some mixture of the two” explanations: some uniquely competent DNA- and RNA-repair proteins paired with these uniquely effective antioxidants protecting them. Battista has largely retired from the D. radiodurans debate but continues to peer review studies on the microbe at the journal Frontiers in Microbiology, where he is an editor.
“I’ve known Michael Daly for decades now,” Battista told Gizmodo. “He’s not the kind of guy that’s going to just put stuff together willy nilly and push it out into the press.” Although Battista is still personally confounded by how exactly the bacteria’s manganese-complexes actually would preferentially protect proteins over lipids (like cell membranes) and nucleic acids (like DNA and RNA), he does think that Daly’s idea could prove to be “a really novel twist on the way vaccines are made.”
“Being perfectly honest with you,” Battista said, “the thing that has bothered me the most about it is, ‘Why would one set of macromolecules be protected and the other not?’” If the complex is simply an antioxidant, reducing the free radical energy of all those highly reactive new hydrogen and oxygen compounds bouncing in and around an irradiated cell, then why wouldn’t it protect every part of that cell? How does this manganese-complex manage to keep the proteome intact, while the DNA and RNA are obliterated?
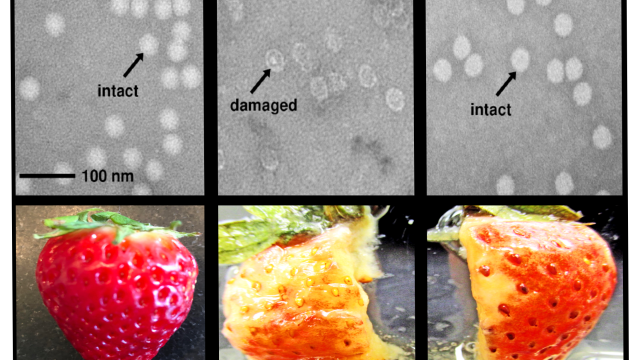
According to Daly, the answer is “not complicated, just chemistry.” One of the major reactive oxygen species produced in cells exposed to gamma radiation, superoxide (O2-), doesn’t chemically interact with either DNA or RNA but does ravage the cellular proteome. Both the intracellular manganese antioxidant complexes found in D. radiodurans (which consist mainly of manganese, orthophosphate, and peptides) and the synthetic version Daly’s team is producing in bulk for their experiments (a manganese-decapeptide complex they call MDP) “only scavenge superoxide efficiently,” Daly says, which means that “MDP protects proteins, not genomes.”
For RNA-based viruses, like coronaviruses in particular, the MDP complexes only interact with the exterior proteins, never penetrating the capsid shell and thus never getting close enough to protect the RNA genome from the inactivating gamma radiation. The result, like an abandoned enemy sub floating out on the open ocean, is a viral husk that drifts inertly through the human body, ready to be captured and studied by the immune system.
Despite billions of dollars in funding from Bill and Melinda Gates, there’s still no reliably effective vaccine for malaria. It killed 405,000 people across the world in 2018, most of them under the age of five, according to the World Health Organisation. Respiratory syncytial virus, a common precursor to both viral bronchiolitis and pneumonia, kills an estimated 250,000 people globally every year. There is no clinically proven vaccine for that, either. There’s no vaccine for the chikungunya, no vaccine for HIV/AIDS, none for hepatitis C.
Theoretically, with just access to a little bit of radiation and some manganese-decapeptide complexes, viable vaccine candidates could be manufactured for each of these diseases, in less time than it takes many vaccine development schemes to even get to a working theoretical model going.
Consider, for example, just some of the punishing 53-year history of the hunt for a malaria vaccine: It began with successfully immunizing mice with an irradiated malaria-causing parasite in 1967 and continued decades later with a U.S. military study, in which 11 volunteers were bitten by irradiated mosquitoes thousands of times, on hundreds of occasions, across the span of the entire 1990s. It still has yet to reach a workable solution. The trial-and-error process for most past vaccines, the breakthroughs and the also-rans, has been, in Tobin’s words, “excruciating.”
Some of the more challenging vaccine experiments carried out with Daly’s concept have proven to be bacterial vaccines — like one for the antibiotic-resistant hospital plague Methicillin-resistant Staphylococcus aureus (MRSA), investigated in partnership with Sandip Datta’s group at NIH in 2012. Preserving too many of the bug’s irritating surface proteins, to make a long government-funded, multi-agency collaborative project’s findings short, goosed immune systems into producing too many detrimental inflammatory cytokines in some of their animal studies.
“There’s something about MRSA, in particular, that we don’t fully understand,” Datta said, “where the immune response has to be just right, and if you trigger the wrong sort of immune response, you can actually make patients worse.”
“A much more logical target for using the manganese-complexes is actually a virus,” he said, “and the reason for that is that viruses are much smaller, practically just nucleic acids. They don’t have the various other inflammatory components, proteins and lipids, that bacteria do.”
In addition to their promising vaccine work on two strains of polio, Daly and Tobin’s groups have already succeeded in making irradiated vaccines that have been highly protective in animal studies for two other RNA-based viruses: Venezuelan equine encephalitis virus (VEEV) and the aforementioned tropical disease chikungunya.
Much as it’s harder to hit a small target than the broad side of a barn, viruses with smaller genomes require more gamma radiation exposure to zap them into lifeless, vaccine-ready husks than species with larger genomes. Coronavirus happens to be about three-times the size of VEEV, chikungunya, or polio (its single-strand of RNA is about 26 to 32 kilobases in length, judging from recent studies), meaning that inactivating it in a bath of aqueous manganese antioxidants and cosmic rays will likely be easier, gentler on its proteins, and less radiation-intensive, than Daly and his colleagues’ past projects.
“Gamma radiation is produced by cobalt-60, which is a by-product of the nuclear industry,” Daly said. “It’s very expensive; it’s very dangerous. It has to be maintained at highly secure facilities — and that was a reason to begin thinking about, well, are there other forms of radiation that might be able to do something similar, but might be much more accessible?”
For their polio work, Tobin said it took only 30-second exposures to ultraviolet light to do the job, compared to the two-to-four weeks historically required for old-school inactivated vaccines (i.e. killing the virus in a chemical bath with formaldehyde).
Rapid-response vaccines are only one of the more timely applications for the antioxidant complexes that D. radiodurans produces. The ability to defend lifeforms against intense radiation has some pretty far-reaching, literally interstellar, implications: If these manganese complexes could help D. radiodurans tagalong with probes to Mars, for example, why can’t they help Homo sapiens travel through space as well?
In 2016, with funding from the Pentagon’s Defence Threat Reduction Agency and the Armed Forces Radiobiology Research Institute, Daly’s group at USU found that their synthetic manganese-decapeptide antioxidants were not only nontoxic in rodents, when either ingested or injected, but that they dramatically protected them from cosmic radiation. Mice treated with the MDP complexes survived gamma radiation exposure up to 9.5 Grey (5 Grey would kill the average human within 14 days.), in comparison to a control group where more than half the untreated mice died within a month.
The goal of this research was to explore how these manganese-decapeptides might one day help protect cancer patients from the ravages of chemotherapy, giving the human body’s own DNA- and RNA-repair mechanisms the cover to operate.
Having followed Daly’s research over the years, Dea Slade — a medical biochemist at the University of Vienna who, like Battista at LSU, has past experience studying D. radiodurans — feels personally most hopeful about these cancer-treatment applications.
“What excites me the most,” she told Gizmodo, “is the potential of this technique in revealing relative radiation sensitivity of cancer cells, based on their manganese-complex content.” Slade thinks that an early, non-invasive search for the total amount of various manganese complexes in a tumour might help doctors decide whether radiation therapy would be effective on a patient-by-patient basis.
“I left this field six years ago, and have only sporadically kept in touch with the developments — primarily in this area of manganese complexes,” Slade said. “It’s perhaps the only area in Deinococcus biology worth pursuing right now.”
So, what exactly is stopping this technology from resolving our very pressing vaccine needs ASAP? Obviously, all of Tobin’s work at BMI is still only in the preclinical development phase. Multiple phases of human trials will be needed. But in Daly’s opinion, it would be ideal if more academic researchers and pharmaceutical companies started looking into the process and trying it out for themselves.
“MDP-complex is very easy to make,” Daly said. “It’s not a trade secret.” Plus, most major universities across the world do already have gamma irradiators. “If they have laboratories where they can start growing the corona, then they would be very much in a position to test these inactivated preparations on animals,” Daly said. “Anyone can use our approach. It’s all been published.”