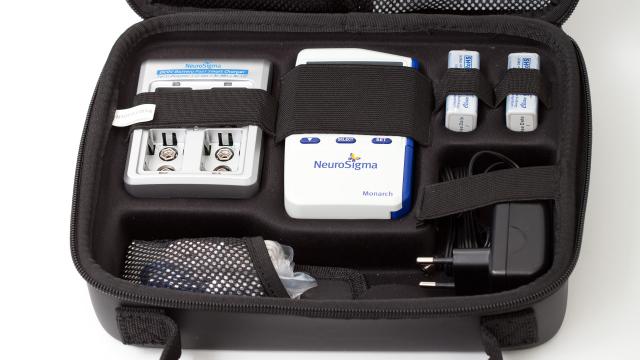
Last week, the US Food and Drug Administration cleared the Monarch external Trigeminal Nerve Stimulation (eTNS) system, a medical device that provides mild brain stimulation to treatment attention deficit hyperactivity disorder in children ages seven to 12.
Judging by the reader emails Gizmodo got about our article on the device, it’s clear there are plenty of misconceptions and concerns about using neurostimulation to treat ADHD. One reader, via email, compared it to electric shock therapy (more formally known as electroconvulsive therapy, or ECT), and feared that it would destroy the brain cells of children who used it.
“IT IS BARBARIC. The public needs to know this before their children’s brains are fried,” the reader wrote.
There’s a few things to unpack here. One is the stereotype that the current form of ECT is especially dangerous.
It’s true that in the past, ECT was performed (and sometimes forced) on patients using high amounts of electricity that would pass through the brain, and that this could seriously harm people and cause muscle convulsions so powerful they would break bones.
But nowadays, people undergo ECT while completely sedated and with safer, smaller doses of electricity, avoiding the possibility of severe convulsions.
ECT still has side effects, including potential memory loss, and it’s only considered best for people with depression or bipolar disorder who haven’t responded to other treatments — but it doesn’t cause brain damage when properly used.
Leaving that aside, the Monarch eTNS system is nothing like even modern-day ECT, according to James McGough, a clinical psychiatrist at the University of California, Los Angeles.
McGough, who is also the chief of staff at UCLA’s Resnick Neuropsychiatric Hospital, and his team studied the Monarch system in the pivotal clinical trial that led to the FDA’s approval of the device. He told Gizmodo that he has no financial relationship with Neurosigma; he received a grant from the National Institutes of Health to study the device.
While the FDA has judged the device to be safe, the agency noted in its clearance that it does have some adverse effects, including drowsiness, an increase in appetite, headaches and fatigue.
McGough said the device’s two wires, attached to a bandage-like patch on the forehead, provide something of a light tingling sensation. Children who use the device likely won’t even experience that tingle much of the time, since the device is intended to be worn while sleeping for about eight hours.
“If you use a tuning fork and put it to your forehead, you’d get more of a vibration than you would from this. We’re not plugging people into a wall socket, we’re using a 9-volt lithium battery to generate a charge over eight hours — it’s so minimum,” he said.
“But that stimulation to your forehead actually feeds back and adjusts the [brain activity of] the areas of the brain that are involved in ADHD.”
As for who might be best suited to use the treatment, the FDA cleared the device — via prescription — for children between the ages of seven and 12 who aren’t currently taking any prescription medications.
Most people with ADHD who rely on stimulants (the first-line ADHD treatment) such as Ritalin do respond to them, but about a quarter don’t. Those patients sometimes use other, non-stimulant drugs, such as the selective norepinephrine reuptake inhibitor atomoxetine.
Monarch’s effectiveness at reducing ADHD symptoms, on average, was similar to the effect size seen with non-stimulant drugs, which are roughly half as effective as stimulants.
Still, for children who don’t respond to ADHD drugs, can’t tolerate their side effects or whose families would prefer to avoid medication, the Monarch might be an option.
For some children, McGough said, the four-week regimen may improve symptoms enough that they don’t need any further treatments. But he also envisions a future in which doctors could prescribe the device in conjunction with medication, as an off-label use, to more effectively manage ADHD symptoms.
Though McGough is optimistic about the future of the device for the treatment of ADHD, both he and his principal co-author Sandra Loo, a professor at UCLA’s Brain Research Institute, say there’s still more research that needs to be done before it becomes widely adopted by doctors.
Key among these future studies would be finding the ideal patients who best respond to the treatment. In their study, about half of children felt some improvement after using the device.
“We plan to conduct further research to identify measures that are predictive of TNS response,” Loo told Gizmodo via email. “I think it makes sense to do this before regular use of TNS.”