Zack Geballe spent months screwing together pairs of polished diamonds at the Carnegie Institution for Science’s Geophysical Laboratory. Theory predicted that squeezed between the diamonds’ tips could be one of the most miraculous substances of modern physics — a material that, at near room temperature, could transport electricity without losing power.
He just needed to get the samples to Argonne National Lab in Chicago to heat them up with laser pulses.
When Argonne beam line scientist Yue Meng turned the lasers on, all four diamonds cracked in half.
“It was a total catastrophe,” Geballe told me while I was visiting him at the Geophysical Laboratory in Washington, DC, this year.
But things have turned around in the past year; two competing teams of scientists have measured near-room-temperature superconductivity in a material called lanthanum hydride.
Their success realises the efforts of over a century of theories, experimental results, disappointments, and cracked diamonds. Nonetheless, their achievement is just one small advance from nearly 110 years of scientific development
Superconductors are materials that can transmit electrical charge without any resistance—unlike a copper wire, for example, which heats up from passing electric current, weakening the transmitted signal. Superconductors have found an important use generating the intense magnetic fields required by MRI machines and high-energy particle physics experiments, but they must be kept at temperatures far colder than those we naturally experience on Earth.
Superconductors haven’t seen widespread commercial applications due to their cost, the effort required to produce them, and perhaps reluctance by old-school companies to adopt such a radically new material, reports IEEE Spectrum.
But a room-temperature superconductor could drastically decrease energy costs and might end up in new technologies that scientists haven’t even dreamed of yet.
Now feels like a turning point: lanthanum hydride is the closest a room-temperature superconductor has felt to reality. But visiting with Geballe at the Geophysical Laboratory, it was hard to imagine the slivers of the material—smaller than the width of a human hair—fashioned into a wire or used in any technology at all. Nor is that the point.
Materials scientists are working at the boundary of the present and the future, performing gruelling, hands-on research hoping to develop substances that might not even have any applications.
“Who knows?” Geballe told me when I asked whether we’d ever see high-temperature superconductors that can exist without being squeezed between diamonds. “Maybe next year, maybe never.”
Crushing hydrogen (and hopes)
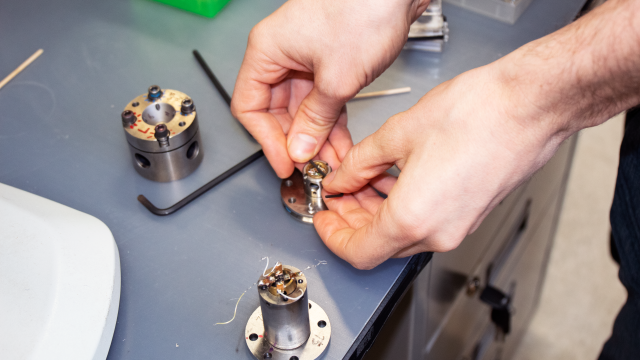
Many of the tools used to create lanthanum hydride can fit in the palm of your hand, and many were already set out on a lab bench when I’d arrived at one of the brick buildings on a hill accented by flowering pink magnolia trees on Geophysical Lab’s campus.
A pair of interlocking steel cylinders slightly larger than D-cell batteries each contained a diamond, point facing upward, at their tops. The points were polished into flat surfaces less than a tenth of a millimetre wide.
When the experiment works as planned, one of the researchers carefully sandwiches lanthanum foil and hydrogen gas in between the diamonds’ flat surfaces. Then, by merely twisting a pair of screws with wrenches held in each hand, the researcher generates pressures of at least 170 GPa—pressures similar to those in the Earth’s core—between the diamond tips.
Then they bring the compressed diamond anvil cells, as they’re called, to Argonne National Lab in Illinois. That’s where the big science happens. Argonne scientist Yue Meng helps the team heat the material with laser pulses, producing the chemical reaction that would create the material.
Geophysical Laboratory x-ray scientist Maria Baldini, now at Fermilab, then helps measure the material’s crystalline structure using the x-rays from a pipe branching off of the 1,103.99m-round Advanced Photon Source particle accelerator to confirm that they’d successfully synthesised the material.
And that’s just creating the material—they’d still need to fit electronic components onto a diamond’s surface in order to measure whether they’d created a superconductor. Plus, they’d need to heat a sample without cracking the diamonds. At these high pressures, the diamonds really want to crack.
“We’re at the point of no return,” Maddury “Zulu” Somayazulu, associate research professor now at George Washington University, told Gizmodo. “Once you take the diamonds to pressures above a million times [the Earth’s atmosphere at sea level], they’re not going to survive. A lot of times, what would happen is we would synthesise a material at the Argonne lab, come back to Geophysical Lab very happy, and find out that the diamond is cracked.”
The first superconductors predate penicillin, television, or the transistor on which computers are built.
They were created without diamond anvil cells, lasers, or particle accelerators. Dutch physicist Heike Kamerlingh Onnes discovered superconductivity in 1911, in the element mercury held to -269 Celsius, just a few degrees above the temperature at which matter has no heat.
Theorists John Bardeen, Leon Cooper, and John Robert Schrieffer finally developed a theory, now called BCS theory, to explain this behaviour in 1957, based on quantum mechanical effects in the materials’ electrons. Frustratingly, scientists would observe superconductivity vanishing in the presence of strong enough magnetic fields or at higher temperatures.
Separately, theoretical physicists Eugene Wigner and Hillard Bell Huntington theorised in 1935 that under high enough pressure, hydrogen would become a metal. Physicist Neil Ashcroft realised in 1970 that this metallic hydrogen might be a high-temperature superconductor and, later, that materials containing mostly hydrogen plus another element mixed in, called hydrides, might also be high-temperature superconductors.
Only after all these scientific advances did physicists have all the ingredients to produce the Holy Grail of materials science.
The impacts of this work wouldn’t fully materialise until the 2010s. Both experimental method (like the tools required to crush hydrogen under immense pressures and make electrical measurements of the material) and theory (like which hydrogen-rich materials the physicists should pursue) would need time to catch up.
For much of the late ‘80s and ‘90s, the records for highest-temperature superconductors were instead held by “cuprates,” copper-containing materials that don’t seem to follow the rules of the BCS theory.
But cuprates were brittle and their behaviour was difficult to predict, Somayazulu explained, and hydrides started catching up. By the start of the 2010s, theorists offered a bounty of tantalising promises to physicists hunting for near room-temperature hydride superconductors.
They predicted that calcium hydride would become a superconductor at temperatures experienced on a cold Chicago day, though researchers could only attain superconductivity in the hydride silane at a temperature only a bit warmer than absolute zero in 2008.
Then in 2014, a team led by Russian physicist Mikhail Eremets would blow the field open by demonstrating superconductivity at temperatures a few hundred degrees above absolute zero by compressing hydrogen sulfide gas.
In 2015, they showed the compressed gas becoming a superconductor at temperatures sometimes experienced during the Antarctic winter. It was a revelation.
A miracle in a gas
Once a department head at the High Pressure Physics Institute of the Academy of Sciences in the Soviet Union during the late Cold War, Eremets later travelled to universities around the world researching high-pressure physics. He eventually went on to lead his own group at the Max Planck Institute for Chemistry in Germany. He was hoping to crush hydrogen. “Everything was related to metallic hydrogen,” he told Gizmodo by phone.
Eremets is a veteran atom-crusher. “I focus on some very difficult problem, and try to solve it by any means,” he said. “I cannot stop until I solve it.”
The hydrogen sulfide gas Eremets’ team was compressing isn’t an exotic material—it’s a rotten egg-smelling gas found throughout the Solar System and produced in our own bodies. “It was available,” Eremets explained. It’s fairly easy to handle. After compressing it to pressures nearly a million times that of the air you breathe, and cooling it to -70 Celsius — again, think cold Antarctic winter — signs of superconductivity emerged. The resistance dropped to zero and magnetic fields didn’t pass through the sample—a crucial signature of superconductivity.
It was almost magical. Hydrogen sulfide was supposed to take on superconductive properties, but not until temperatures dropped much lower than those observed. The team theorised that under high pressure, some of the hydrogen sulfide atoms had taken on extra hydrogen atoms, bestowing them the higher transition temperature.
High-pressure hydrides have finally begun to show off their theorised promise as high-temperature superconductors. And the race was on to synthesise ones whose resistances dropped at even higher temperatures — perhaps even at room temperature.
The race begins
Back in the United States, influential high-pressure scientist Russell Hemley (now at George Washington University) assembled a hydride-hunting team of his own. Then based at the Geophysical Lab, it included Somayazulu, superconductivity researcher Muhetaer Aihaiti, beamline and x-ray scientist Maria Baldini, and theorists Ivan Naumov and Hanyu Liu, with the help of scientist Yue Meng at Argonne and later joined by post doctoral researchers Ajay Mishra and Zach Geballe.
Somayazulu, like Eremets, had a long history of attempting to crush hydrogen, but after many failed attempts had begun researching hydrides, while Aihaiti had previously researched superconductivity in cuprates before working in high-pressure physics.
Guided by Liu’s theoretical predictions, the team worked to feed hydrogen into diamond anvil cells with pieces of yttrium or calcium wedged between the tips. After many tries, Mishra failed to synthesise the crystalline structures that the computer algorithms predicted. They set their sights on lanthanum hydride, and the task of producing that material would fall mostly to Geballe.
“By then, we had had all become quite exhausted breaking all these diamonds. These were very difficult loads to do,” Somayazulu explained. “But [Geballe’s] experiment was the spark that energised us. It was right on the money.”
I met up with Geballe at the Geophysical Lab campus in northwestern Washington, DC. The scruffy, lanky physicist hadn’t planned on hunting for superconductors—his research focused more on developing new methods to measure how high-pressure materials stored heat and how matter behaves deep within the Earth. But he was wooed by the idea of working on hydrides, especially under Somayazulu’s lead.
“It was pretty special that someone with so much experience had the devotion to young people who don’t know what they’re doing,” Geballe said. (He also happens to be the grandson of another well-known superconductivity researcher, Ted Geballe.)
Like the calcium and yttrium hydrides before it, lanthanum hydride proved to be a slog. Even once the sliver of lanthanum foil and hydrogen gas were wedged between the diamond cells, the team would need to find a way to insulate the material from the outside world, and to place a crosshair of wires onto the diamond tips that would touch the lanthanum hydride crystal on four sides so they could measure whether it turned into a superconductor. But after months of effort, they simply couldn’t find the signature in their x-ray measurements showing that they’d successfully created the material.
Then, Somayazulu remembered something from his past experiments. He’d hoped that a material called ammonia borane could become a useful hydrogen storage tool. But instead, it released hydrogen atoms under pressure. Rather than hydrogen gas, ammonia borane would offer the hydrogen atoms that the experiment needed in a more controllable way.
This still wasn’t an easy substance to work with: It’s a highly reactive, flaky white powder that absorbs moisture right out of the air. You have to handle it in a glove box with the atmospheric air removed, lest you take another unsuccessful trip to Chicago. But it supplied the necessary hydrogen.
Said Somayazulu: “We put two and two together, we tried it, and it worked.”
All they had to do was bring these samples to Chicago to heat the squeezed lanthanum foil and ammonia borane with the special laser-pulsing technique developed by Meng to create lanthanum hydride. A constant, controlled laser beam on the diamonds could crack them, so Meng’s method instead used short laser pulses to heat the sample.
“To control the short pulse is pretty much the key,” she said. Baldini would help them measure the crystalline structure of the outcome to confirm whether or not they’d actually produced the material.
After shattering all of those diamonds, the team finally made lanthanum hydride in June 2017—with pressures slightly below what the initial theories had suggested—and published their results later that year.
After bringing the material back to the lab at the Carnegie Institute, Somayazulu led the effort to successfully measure zero resistance in the material at temperatures higher than -13.15 Celsius, perhaps as high as 7 Celsius — an autumn night in DC. They began working on a paper, hoping to be the first to broadcast their historic findings to the world. The pressure was on.
All this time, Eremets’ lab hadn’t published papers on lanthanum hydride—though the Geophysical Lab team knew that, given how good an experimentalist Eremets was, there was no doubt he himself was working on something important. “We were sure Eremets would reproduce it immediately,” Somayazulu said.
In May 2018, Hemley presented the results at a conference in Madrid, and a reporter for Physics Today magazine began writing a story about the unpublished result.
But on August 21, Eremets’ team’s paper, documenting superconductivity in lanthanum hydride at slightly lower temperatures, appeared on the arXiv physics paper server.
The Washington, DC, team, whose researchers were now split between the Geophysical Laboratory and George Washington University, submitted their paper to the server on August 23, the same day that the Physics Today story came out. Eremets had won the race.
“It was out of order,” said Geballe. “I was disappointed.”
Despite publishing second, Somayazulu, Geballe, and the other researchers from the lab still chalked their results up to a win. “We were very happy that there were two groups showing similar results around the same temperature,” Somayazulu told me. “We independently verified with different techniques of making it. It’s great science.”
Aihaiti at the Geophysical Lab put it differently—the teams require one another to exist, regardless of who publishes first. “Someone else has to prove it independently, otherwise it’s invalid,” he told Gizmodo. You don’t win the Nobel Prize for a result that can’t be replicated, after all. And each member of both teams provided a crucial piece, without which the discovery would never have been made.
For all the effort, it’s hard to say whether we’ve gotten much closer to a reality of high-temperature superconductors. Lanthanum is just another material from a list of potential superconductive hydrides that theorists’ programs spat out.
It’s hard to imagine lanthanum hydride superconductors ever appearing in consumer technology. The teams synthesised only about a dust-speck worth of the material from expensive ingredients crushed to unfathomable pressures between hand-cranked diamond halves.
There are yet more experiments to be done on lanthanum hydride in order to validate that it truly does become superconductive at the advertised temperatures, as well as experiments on other materials. Eventually they’ll have to figure out how to turn the pressure down.
The race to produce lanthanum hydride demonstrates the fractal-like nature of science research. Each advance builds on decades of prior knowledge—but even the efforts to synthesise lanthanum hydride were just a small snapshot of the work going on across the world on hydrides.
Another well-known high-pressure physics group from the University of Osaka has joined forces with Eremets’ team to measure the superconductivity of hydrogen sulfide in a high magnetic field. HPSTAR, an institute in China established by pioneering physicist Ho-Kwang (Dave) Mao (once a Geophysical Lab scientist himself) has also joined the effort.
But, while hydrides hold the high-temperature superconductor record today, there’s no telling whether other materials might prove more useful in the future. And there’s an entire field of high-pressure physics studying plenty of other properties in these materials.
But lanthanum hydride is still a critical contribution to the field. By exploring deeper — figuring out why lanthanum hydride turns into a superconductor at ambient temperatures — perhaps theorists like Liu will be able to tweak their codes to reveal other materials that will maintain superconducting behaviours at higher temperatures or lower pressures.
Perhaps improvements to the fabrication process will bring cheaper materials, or maybe we’ll find a way to get high-pressure lanthanum hydride into a wire.
For now, the science progresses slowly, and even these small steps take gargantuan, international efforts. Without the work of both teams, there would be no lanthanum hydrides. Each contributing scientist played an irreplaceable role, and through competition came harmony.
“I’ve never seen this kind of fantastic synergy between theory and experiment. And there are all of these groups—in Germany, Russia, in China, in Japan, and in the United States,” Somayazulu told me, “who are working not only on predicting [superconductors], but modifying current experimental techniques to do these really challenging measurements. It’s bringing us all together, and we’re forming different alliances and groups of scientists to do it. That’s what’s great about these results.”