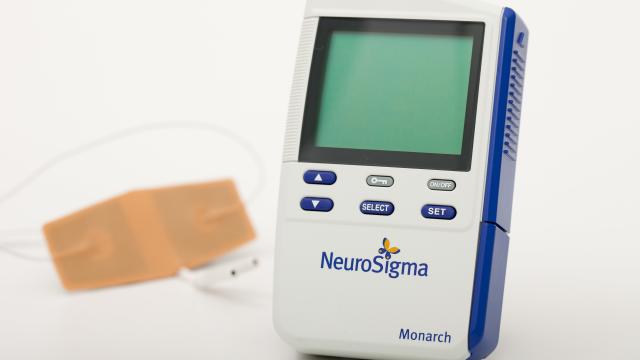
Families of children with attention deficit hyperactivity disorder (ADHD) will now have a new, non-drug, treatment available to manage the condition: a medical device attached to the forehead that sends mild shocks to the nervous system.
Over the weekend, the Food and Drug Administration announced they granted clearance of NeuroSigma’s Monarch external Trigeminal Nerve Stimulation (eTNS) System for children with ADHD. (The FDA does not generally “approve” medical devices the same way it does with drugs).
The treatment will be intended for those ages 7 to 12 who are not currently taking any other prescription drugs for their ADHD. But it will also only be available via prescription.
“This new device offers a safe, non-drug option for treatment of ADHD in pediatric patients through the use of mild nerve stimulation, a first of its kind,” Carlos Peña, director of the Division of Neurological and Physical Medicine Devices in the FDA’s Center for Devices and Radiological Health, said in a statement.
There’s a wide variety of treatments that are used to help manage ADHD in both children and adults, a complicated disorder that involves symptoms like a constant inability to pay attention, impulsiveness, trouble sleeping, and mood swings.
They range from behavioural therapy to stimulants like the brand name Adderall and Ritalin to the selective norepinephrine reuptake inhibitor (SNRI) atomoxetine. These treatments all work to an extent, particularly stimulants, in that they’re better than a placebo overall. But not everyone benefits from them.
For instance, about 20 to 25 per cent of children with ADHD don’t respond to stimulants, according to a 2016 review. For these cases, atomoxetine is usually effective, but it has its own side-effects, including short-term suicide ideation. And even when stimulants do work, they can come with serious side-effects, including the future risk of addiction and substance use disorder.
Nerve stimulation has emerged in recent years as another way to manage behavioural and neurological conditions like depression, epilepsy, and anxiety. There are several forms of it — including one that requires implants surgically placed into the brain — and we’re still unclear about its exact mechanisms, but the essential gist is that nerve stimulation to the right places can temporarily quiet the erratic brain activity associated with a specific condition.
In the case of the Monarch eTNS system, the shocks are sent from a small, smartphone-sized device to wires that are attached to a patch on the forehead right above the eyebrows. The shocks then go along the trigeminal nerve, the cranial nerve that’s largely responsible for our sensations of biting and swallowing, which then directly or indirectly reach parts of the brain, like the cerebral cortex, that help regulate our emotions, behaviour, and ability to focus.
The key piece of evidence used to convince the FDA was a double-blinded, randomised and controlled trial of 62 children with moderate to severe ADHD, which was published earlier this January. Those who were given the real device over a four-week period every night had a significantly greater reduction in their symptoms (as judged by a clinician) than those given a non-functioning device.
According to the study’s authors, the degree of symptom improvement seen in these children was about the same you’d expect to see from those given any non-stimulant treatment, which tend to be slightly less effective than stimulants. But one real advantage the Monarch seems to have over stimulants is the lack of any serious health risks. Side-effects did include drowsiness, an increase in appetite, trouble sleeping, teeth clenching, headaches, and fatigue.
Safe as the device is, it’s intended only to be used with the supervision of a caretaker. The treatment also takes about four weeks before symptoms reliably improve. And children with pacemakers or other devices implanted in the body are not recommended to use it.
Colin Kealey, vice president of Advanced Development & Medical Affairs at NeuroSigma, told Gizmodo via email that there is no specific date as of yet for the Monarch’s launch in the U.S., though they do hope to have it available soon.
“We anticipate the price of the Monarch eTNS System will be in-line with the current price of brand name ADHD medications,” he added. “We do not anticipate insurance coverage in the early weeks after launch, however, securing insurance as soon as possible is a high priority for NeuroSigma.”
The system was already approved for the treatment of ADHD, epilepsy, and depression in the European Union, but this would be its first approval for any condition by the FDA.
“As a general rule we do not comment on specific plans, but NeuroSigma may consider running additional clinical trials to support submissions to the FDA for label expansion and to request clearance for other indications,” Kealey said.