Even under the best of circumstances, cancer treatment can be an excruciating, costly ordeal that tragically doesn’t even work sometimes. In light of that reality, scientists and doctors have long searched for a way to proactively head off the problem using a vaccine. One potential approach to a cancer vaccine, highlighted in a new study published today in Cell Stem Cell, might involve using our own reprogrammed stem cells to better train the immune system against several – and maybe even all – types of cancers.
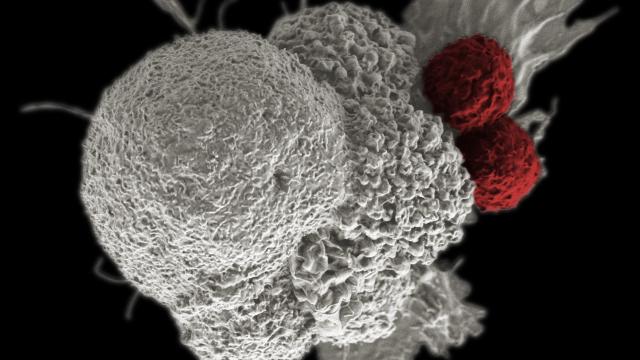
Above, a cancer cell (white) being attacked by T-cells (red). Image: National Institutes of Health
Researchers at Stanford University injected mice, which were specially bred to develop a multitude of tumours, with induced pluripotent stem cells, or iPSCs. iPSCs are adult cells which have transformed into stem cells that behave remarkably similar to those found in embryos – capable of becoming virtually any other type of cell needed. Thanks to their expansive expression of the genetic code that every cell is programmed with, scientists have also long theorised that embryonic stem cells could sensitise the immune system against the proteins, or antigens, responsible for sparking the uncontrollable growth that defines cancer.
The major stumbling block, however, has been that scientists (more accurately, the governments and companies that fund them) have been squeamish about experimenting with stem cells taken from embryos in any capacity. With the advent of the technology that makes iPSCs possible – first demonstrated in 2007 – the researchers, led by Joseph Wu, director of the Stanford Cardiovascular Institute, finally felt secure in testing out the stem cell theory.
Across different experiments, they found, the mice given iPSCs (the stem cells were first killed via radiation) were shown to be better at fighting off cancers of the breast, lung and skin. One experiment revealed that giving untreated mice a dose of immune cells taken from the iPSC-treated mice bolstered their defences too and reduced tumour growth. Another experiment showed that the vaccine helped mice whose cancers were removed surgically better avoid a relapse.
“This is absolutely a fascinating study and adds more evidence that cancer stem cells could be good antigen targets for cancer vaccines,” Sasha Stanton, an oncologist at the University at Washington who is unaffiliated with the research, told Gizmodo.
Wu and his team believe the potential of an iPSC-derived cancer vaccine could be tremendous, if still unknown.
“We’re describing a true vaccine. It’s not something you start treating a patient with if they already have cancer,” Wu said, though he notes it could also be used to help prevent cancers from returning or spreading elsewhere in survivors. “You would already have an immune system that is activated to target these potential cancers before they form.”
Currently, doctors can take out a person’s T-cells, modify them in the lab to recognise and go after a specific cancer cell antigen, then put them back in the person, where they hopefully begin to multiply en masse and do their newly assigned job of attacking cancer cells. This form of immunotherapy, known as CAR T-cell therapy, has already been used to successfully treat children with aggressive, recurring leukaemias that might have otherwise been incurable. But it’s also a very intensive and even potentially fatal option that sometimes still fails, either because the T-cells can lose their reprogramming midway through or the tumour cells themselves mutate. That’s why it’s been reserved as a last-resort treatment.
The treatment developed by Wu’s team on the other hand seems capable of both passively training T-cells to recognise a broad array of tumour antigens as well as the body’s B-cells, which are responsible for generating antibodies. That in theory could lead to a sustained, safer immunity against thousands of different cancer-related antigens, even those we don’t know exist yet.
“It wouldn’t surprise me if this vaccine worked on a large amount of cancers, if not all cancers,” Wu said. “That’s something we need to figure out in the next few years.”
This multi-pronged technique might not only provide a buffer against many cancers, Stanton said, but could also prevent cancer cells from evading the immune response as they can with CAR T-cell therapy. The use of iPSCs, so long as they’re sourced from the person themselves, would allow for a customised vaccine that would lessen the risk of incompatibility, Wu added.
“However, in moving to study this in human beings, care has to be taken in putting pluripotent stem cells (even irradiated cells) into people,” Stanton said. “There is always a risk that these cells could survive and induce the development of a cancer or cause an autoimmune response against a non-cancer stem cell target that could harm the patient.”
So far, Wu and his team have been diligent in looking for potentially disastrous side-effects. “Some of these things we did, obviously, was look at the mice during the vaccination procedure to see if they showed any signs of auto-inflammatory response,” he said. “We did not see anything like that in our study. These mice are thriving. They’re eating well, gaining weight – they look good.”
No matter how impressive animal results might be, though, the real challenge will be showing them in humans. Wu and his team are already gearing up to try out their vaccine in the lab on human cells. And provided that goes smoothly, they would then take their first steps toward small human trials in the years to come.
Other cancer vaccines are in varying stages of development. Stanton and her team at the University of Washington are themselves working on a vaccine that relies on breast stem cells, while a separate team at Stanford is recruiting patients with low-grade non-Hodgkin lymphoma to test out a localised, therapeutic vaccine that would come with fewer side effects than CAR T-cell therapies.