In a scientific first, researchers from the US and the UK have taken early-stage human egg cells and grown them to full maturity in the lab. It’s an important proof-of-concept that could eventually yield new infertility treatments for women.
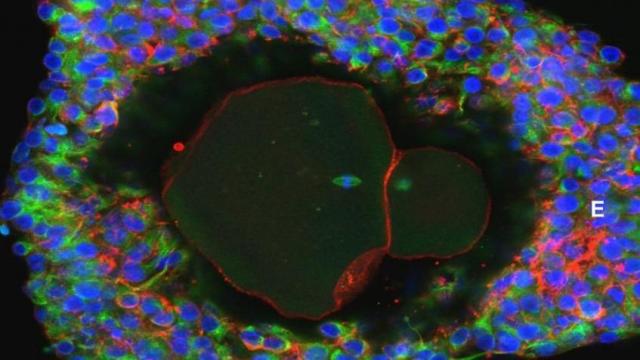
A lab-grown fully matured human egg ready for fertilisation. (Image: David Albertini/University of Edinburgh)
Growing egg cells to full maturity in the lab isn’t anything new. Scientists have already done this with mice, where lab-grown oocytes, or developing female sex cells, have resulted in the birth of live offspring. Also, human eggs have been grown to full maturity in the lab before – but from cells that were already at a late stage of development. What makes the new study special is that it’s the first time scientists have achieved this with early-stage human eggs cells, setting the stage for fertilisation by sperm – at least in theory.
Developed by researchers from the University of Edinburgh and the Center for Human Reproduction, the technique could eventually be administered to women whose eggs don’t develop fully in their bodies, and as a way to preserve a cancer patients’ ability to have biological offspring in cases where chemotherapy or radiotherapy has made them infertile. Conceptually, this breakthrough means a girl’s immature eggs can be recovered from her ovarian tissue, matured in the lab, and then cryogenically stored for future in vitro fertilisation. Currently, a piece of ovary is removed before chemotherapy and then re-implanted after the treatments are done, but this introduces the risk of putting cancer cells right back into a patient’s body.
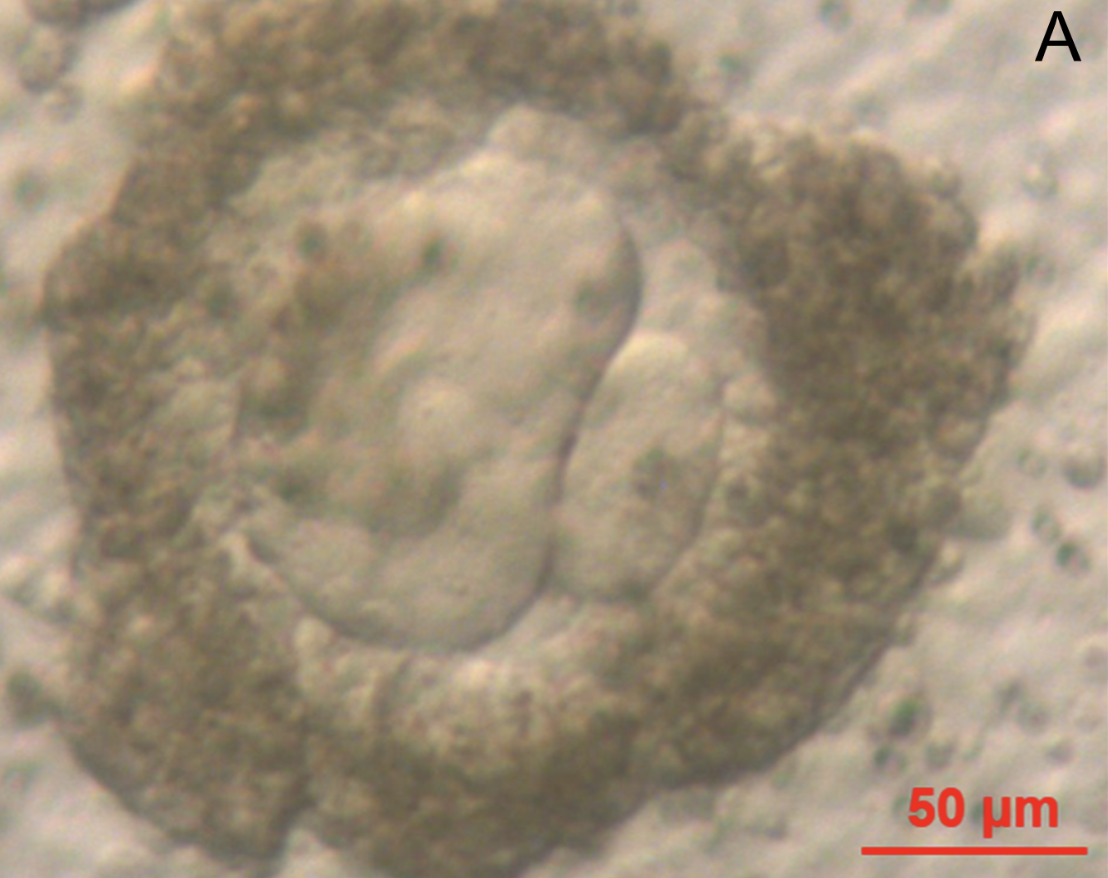
Microscopic image of the finished product. (Image: M. McLaughlin et al., 2018)
The key to the study, published today in Molecular Human Reproduction, was the development of effective culture mediums – environments in which the immature cells could be supported through each stage of development and brought to full maturity. In experiments, the researchers extracted ovarian tissue from 10 women during routine surgery, all of whom were in their 20s or 30s. The cells were bathed in a series of complex chemical nutrients over the course of four stages of development. Nearly 50 eggs of out 87 attempts managed to reach the third stage, with nine making it through to full maturity.
“This is a small number of samples but provides proof of concept that complete development of human oocytes can occur in vitro,” wrote the authors in the study. “Further optimization with [physical] evaluation and fertilization potential of [lab grown] oocytes is required to determine whether they are normal.”
Early indications suggested the mature eggs were in fact normal, but there was some weirdness during growth. For an unknown reason, the eggs developed faster than they would have inside the body, and one type of cell involved in the process – a polar body – grew to an unusually large size. Something may be a bit off, so further research will be required.
“Being able to fully develop human eggs in the lab could widen the scope of available fertility treatments,” study co-author Evelyn Telfer from the University of Edinburgh’s School of Biological Sciences said in a statement. “We are now working on optimising the conditions that support egg development in this way and studying how healthy they are. We also hope to find out, subject to regulatory approval, whether they can be fertilised.”
The method represents an important breakthrough, but it’s also important to not get ahead of ourselves. The eggs could very well be abnormal, or unable to support normal fertilisation and embryonic development. Subsequently, it may be a while before we see this technique used in clinical settings.
Speaking to The Guardian, Francis Crick Institute scientist Robin Lovell-Badge said it was possible that some of the early-stage egg cells used in the experiment may not have been as immature as the scientists believe. And Ali Abbara from Imperial College London told Reuters that “Much more work is needed to make sure that the technique is safe and optimised before we ascertain whether these eggs… can be fertilised.”
All that said, the new technique is demonstrating some new things about egg development that scientists didn’t know before, and it could inform research into other infertility treatments and regenerative medicine. It’s still early days, but hopeful ones nonetheless.