Photo: Getty
EpiPen manufacturer Mylan has been involved in a contentious legal case over its overcharging of the US government. On Thursday, the US Department of Justice finalised its settlement with Mylan, which will pay $US465 ($589) million in penalties. But many believe that’s not enough.
[referenced url=”https://gizmodo.com.au/2017/06/us-senator-epipen-manufacturer-ripped-off-the-us-government-for-1-7-billion/” thumb=”https://i.kinja-img.com/gawker-media/image/upload/t_ku-large/gjlw8w3npyzlrfpk6iny.jpg” title=”US Senator: EpiPen Manufacturer Ripped Off The US Government For $US1.7 ($2) Billion” excerpt=”For people with severe allergies, having an EpiPen can mean the difference between life and death. Because there’s no generic alternative, EpiPen manufacturer Mylan just keeps jacking up the price and ripping off patients. They also seem to have ripped off the US government. Today, a probe by the US Department of Health and Human Services concluded that the company actually stiffed American taxpayers for three times more than was previously believed.”]
According to Reuters, Mylan admitted no wrongdoing by agreeing to the settlement:
The U.S. Attorney’s Office in Massachusetts revealed the accord on Thursday, 10 months after Mylan said it reached a deal resolving claims it misclassified the EpiPen as a generic rather than a branded product to avoid paying rebates owed to Medicaid.
“Taxpayers rightly expect companies like Mylan that receive payments from taxpayer-funded programs to scrupulously follow the rules,” Acting U.S. Attorney William Weinreb said in a statement.
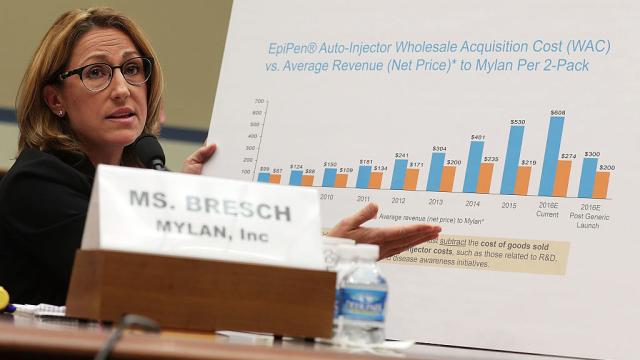
Mylan has been a poster child for the pharmaceutical industry’s greed since it began hiking the price of the life-saving EpiPen allergy treatment in 2008. Over the last decade, Mylan has raised the cost of an EpiPen two pack from around $US100 ($127) to over $US600 ($761). Lacking generic alternatives, patients paid the extortionate price. But the craven executives also reaped the benefits of a government reimbursement program through Medicare and Medicaid. Despite not offering a generic version of its product, Mylan classified the treatment as a generic drug — meaning it only had to reimburse 13 per cent of the total cost paid out by one of the government programs. If the product had been properly classified, that reimbursement amount would have been set at 23.1 per cent.
Mylan initially agreed to the $US465 ($589) million settlement in October, but in May, a probe by the Department of Health and Human Services concluded that the company owed nearly three times that amount. “Mylan and the Obama Administration reportedly were close to settling the overpayment for much less than $US1.27 ($2) billion,” Senator Chuck Grassley wrote in a statement at the time. “Taxpayers have a right to know what happened here and to be repaid whatever they are owed.” Maybe Grassley got distracted by something in the meantime because the deal is done and it appears that Mylan will be off the hook for the other $US805 ($1,020) million.
But that doesn’t mean that things are turning out great for Mylan. A rival company from France called Sanofi SA originally filed the False Claims Act whistleblower lawsuit in 2016 and the company will receive a reward from the government in the amount of $US38.8 ($49) million. That’s small change to these large conglomerates, but it’s a stick in the eye to Mylan as it watches its stock price fall. Sanofi SA sold the rights to its epinephrine injection device Auvi-Q to Kaléo who began selling it as an EpiPen alternative in April. As that and other rival products hit the market, Mylan has introduced its own generic version for $US300 ($380) and seen its market share shrink from 95 per cent to 71 per cent in March. As of August 16th, Mylan’s share price had fallen over 23 per cent in a month.
Following the finalization of the settlement, Mylan Chief Executive Heather Bresch issued a statement saying, “Bringing closure to this matter is the right course of action for Mylan and our stakeholders to allow us to move forward.” But with analysts downgrading the company’s stock and some recommending that investors sell, it’s doubtful that stakeholders are feeling relieved.