Since at least the first time a man got on stage at a TED talk and 3D-printed a human kidney, the idea that one day we might simply grow new body parts to replace our old, out-of-service ones has existed in the collective consciousness as the pinnacle of biomedical achievement. But what if you didn’t actually have to grow a whole new organ to save someone’s life?
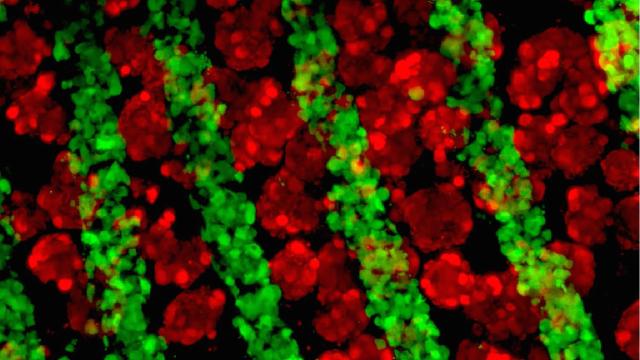
Growing liver cells and connective tissue under a fluorescence microscope. Credit: Stevens, et al. Science Translational Medicine
A paper out Wednesday in Science Translational Medicine details a new method of growing organ tissue in mice that mimics the complicated cellular architecture of the human liver. It also poses an intriguing question: Could a liver engineered to exist somewhere else in the body act as a bridge while a patient waits for a transplant, or perhaps pick up the slack for an ailing liver?
Not only might that be possible, it might mean lab-grown organs make it out of the lab and into the clinic a lot sooner.
“The main goal here is to deliver a liver than can be useful to a patient,” Kelly Stevens, a co-author of the paper and bioengineer at the University of Washington, told Gizmodo. “For that to happen, it might not be that we have to build a whole new liver.”
Stevens explained: “In cancer, for example, we could more aggressively cut out cancerous tissue and then put in a baby liver that replaces that missing piece. That could get the patient through until the liver heals on its own.”
This isn’t exactly a new idea. Making a liver that can successfully pull off a few of the 500 or so functions the liver performs is obviously a lot easier than making one that pulls off all 500. And scientists have long known that, as complicated as the liver is, a pared down knock-off still might be useful.
Stevens and her colleagues, though, count themselves among just a few research groups starting to look at how this might work practically, engineering a standalone liver “organoid” that could be implanted elsewhere in the body. In their view, if your liver failed, maybe you could get a little teeny liver implanted somewhere else in order to supplement it.
“What if a patient is only missing one of the liver’s 500 functions?” Stevens said. “Maybe a baby liver would be enough. The liver is second only in complexity to the brain. But if you could even add back the ability of one function it might be enough to save someone.”
The other advancement in this new work is how the researchers actually went about growing the cells. Typically, when bioengineers want to “make” an organ, they build a “scaffold” that’s approximately the shape of the organ in question, and then they populate it with the right kinds of cells. Nature then takes over, organising all those different types of cells how they’re supposed to go. But in the name of efficiency, the researchers behind the new study built a scaffold that put the cells in the right places that would instead coax the cells to “grow”. They implanted this into the abdominal cavities of mice with liver injuries. Over 80 days, the scaffolds grew into little mini livers that mimicked some features of functional livers. This method, they said, made bigger livers that act like, well, livers. This, too, bodes well for the future of regenerative medicine.
“This research and research like it is crucial to lead to new parts for people,” Dave Gobel, CEO of the Methuselah Foundation, told Gizmodo.
Still, Gobel, who was not involved with the study, noted the research was “very, very early” and still has no clear “near-to-mid term clinical implications”.
And growing full, human-sized, functional organs is still a long way away. Some estimates have suggested this may happen within the decade, at least for simpler organs, but there are still a lot of kinks to work out though. How do you incorporate bile ducts, for example, so the liver can also remove waste? How do you scale it to the size of a human? Not to mention, in Stevens’ research, in order to to implant an organoid into another mouse, the mouse was required to not have an immune system to help it accept the new organ. Obviously, humans have immune systems.
But there are clear steps being taken toward creating something that might actually help people in the near term.
The biotech firm Organovo, for example, is working on a slightly different approach, 3D-printing “patches” of liver tissue to help patients in need of a transplant. Last spring, it announced plans to submit its patches to the FDA for review within three to five years.
“The cool thing about the liver is that it does regenerate itself, so if you could augment a diseased liver by 10 per cent or more, you have a shot a keeping someone alive until a transplant liver becomes available,” said Gobel, whose company has invested in Organovo. “Or in best case, the liver might get enough of a boost to turn the corner and regenerate itself.”
Gobel said he expects within three years we’ll be doing organ augmentation inside the body, something akin to Organovo’s patch system. Stevens said she sees their approach being ready for the clinic within a decade.
“The idea of making a whole new liver is sexy,” said Stevens. “Practically, scientists are thinking about all these other things.”