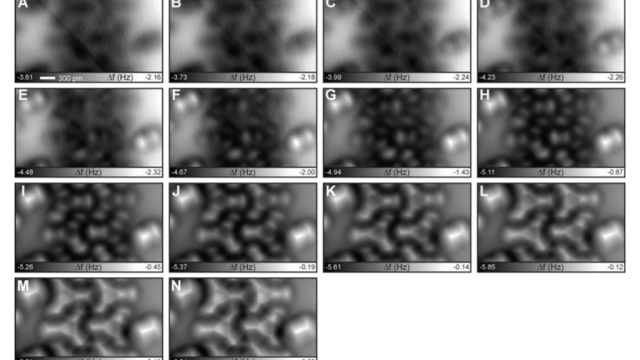
Water has some special properties you’ve surely heard about in high school chemistry. Most notably, it sticks to itself really well. It beads together, looks like this in space, and climbs up plants’ vascular systems, all thanks to hydrogen bonds. Now, scientists have figured out exactly how sticky those ubiquitous bonds are.
More molecules imaged by the researchers (Kawai et al)
[referenced url=”https://gizmodo.com.au/2013/04/even-wringing-a-wet-cloth-is-magical-in-space/” thumb=”https://i.kinja-img.com/gawker-media/image/upload/s—-z-wVCL–/c_scale,fl_progressive,q_80,w_800/18l0zp88oep8tgif.gif” title=”Even Wringing A Wet Cloth Is Magical In Space” excerpt=”Astronaut Chris Hadfield continues to make us all insanely jealous of the time he’s been spending on the International Space Station with another video showing what day-to-day life is like orbiting the Earth. Except this time he shows what happens when you wring a soaking wet cloth in zero gravity, and the results are almost magical.”]
Scientists have imaged hydrogen bonds before, but are now reporting that they have quantitatively measured them in action, using incredibly high-resolution microscopes. And getting a better understanding of hydrogen bonds can be a big deal when studying molecules that rely on them. That includes our own DNA, whose two chains are held together by hydrogen bonds.
Normal bonds occur when atoms share electrons. But hydrogen atoms are just single positive charges attached to single electrons, so when they bond with something else, the resulting molecule has an exposed positive side. This side can stick to negative bits on other molecules. The new technique was able to measure the strength and behaviour of these bonds.
“Potentially, this technique can be expanded for identifications” of the geometry and chemistry “of more complex large molecules such as DNAs and polymers,” they write in the paper published Friday in the journal Science Advances.
There are lots of different ways to observe the various atoms that makes up molecules, including techniques with long names like “infrared spectroscopy”, “nuclear magnetic resonance spectroscopy” and “X-ray crystallography”. These methods require analysing the light that bounces off a sample. But in all the cases, it’s super difficult to spot individual hydrogen atoms.
That’s where the method behind the new imaging — atomic force microscopy — comes in. Researchers have previously used it to analyse individual atoms in molecules, but never down to the resolution of hydrogen. The microscope is essentially a probe with a molecule on the hyper-sharp tip, which in this study was carbon monoxide. The researchers found that the strength of the strongest hydrogen bonds were around 40 pico-Newtons maximum, or roughly 25 billion times smaller than one Newton, which is the force required to accelerate a 1kg mass from zero to 1m/s in one second. In other words, it’s a teeny tiny force, but it’s what they expected.
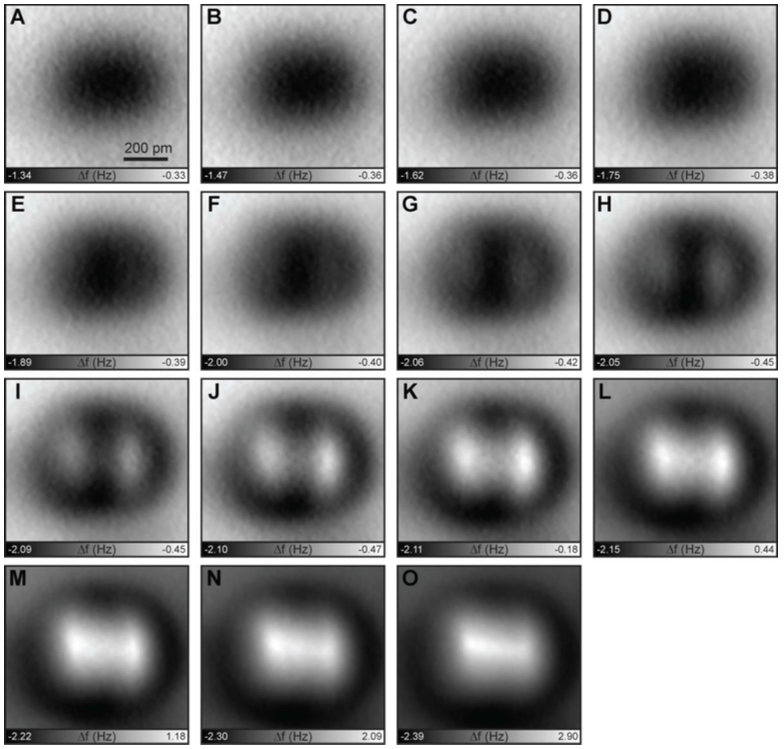
More molecules imaged by the researchers (Kawai et al)
One researcher I spoke with, chemist Henry Rzepa at the Imperial College of London, thought the technique was cool — it “effectively completes the periodic table for the atomic force microscopy technique”. Chemists normally infer the existence of hydrogen atoms in molecules, and their inferences are normally correct. But every so often, inferring the existence of the hydrogens isn’t enough, especially when researchers want to confirm the existence of an unknown hydrogen bond.
Still, “It will help close a small but significant gap in the methods we have for studying the structures of molecules,” he said.