The act of boiling water helps us brew coffee and cook pasta — and it’s also what fuels most of the world’s energy sources. But boiling is really all about the bubbles, and until now their formation had been seen as random and haphazard. MIT engineers say they can now control the formation of bubbles, which might change the way power is generated.
Controlling the way boiling water bubbles, or, in thermodynamic terms, nucleates, might seem — on the surface at least — as a very simple task. But as the engineers explain in their paper, which is published in Nature Communications, it’s an elusive concept that has some incredible real-world applications.
When power plants want water to boil more vigorously there have traditionally been only two options: Crank up the heat by employing more energy, or use a chemical other than water that has a lower boiling point. When you’re talking about large-scale power generation, neither option is really that practical.

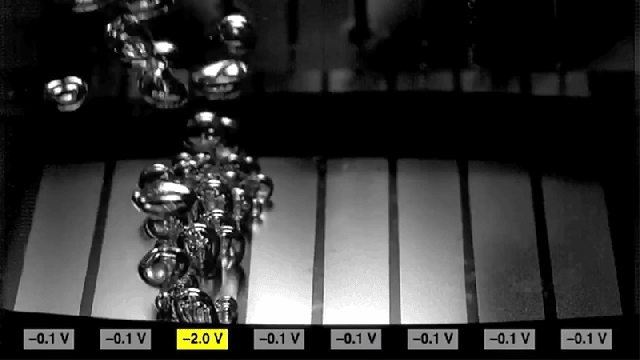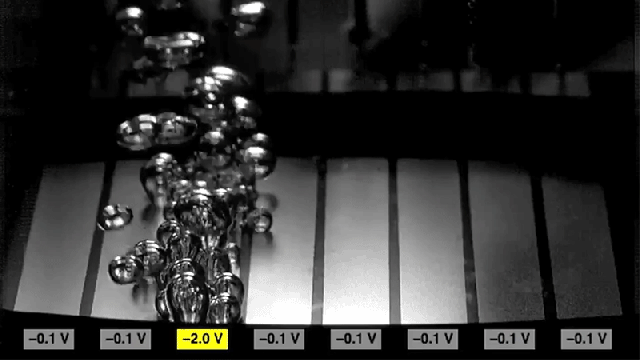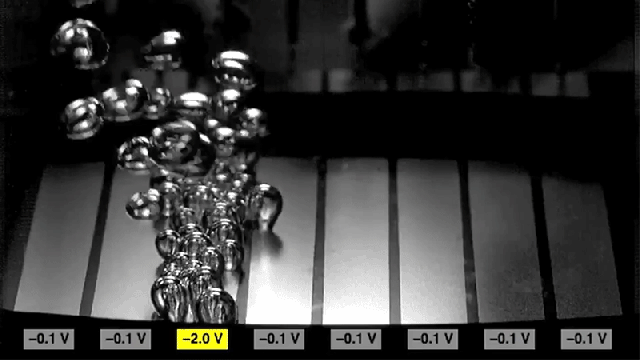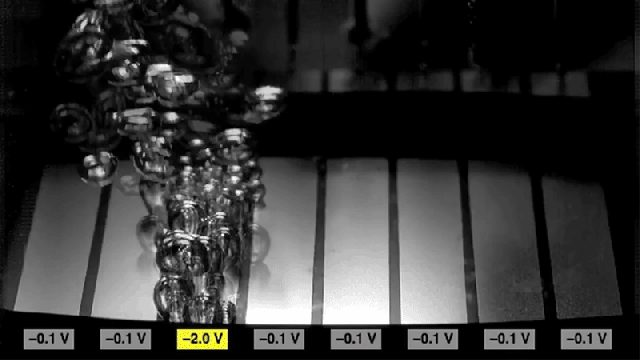
Researchers found that sections of metal can be made to either promote bubbling (the two rectangles at the edges) or to inhibit bubbling (center rectangles), simply by switching the polarity of voltages applied to the metal.
Instead, the researchers tried combining the water with a surfactant, a soapy compound that changes surface tension. Surfactant molecules can be electrically charged but they also have the ability to be both hydrophobic (repelled by water) and hydrophilic (attracted to water). When voltage was applied to the surfactant-infused water to make it boil, the engineers noticed something interesting: The molecules could switch from hydrophobic to hydrophilic, depending on the polarity:
Adding the surfactant causes the surface to become more hydrophobic, which increases the rate of nucleation to form bubbles. But reversing the charge on the surface causes the surface to become hydrophilic, and inhibits the formation of bubbles. The researchers found that they could achieve a tenfold change in the rate of bubble formation simply by switching the charge.
You’ve probably seen how this would work when you’re boiling water for pasta. Bubbles first start to form around any kind of irregularity in the water, perhaps around a scratch in the metal of your pot or the grains of salt you’ve tossed in. In this case, the hydrophobic molecules create that same irregularity in the water, increasing the rate of nucleation. So essentially water could be made to “boil” at the flip of a switch, when the proper charge is applied to the surface.
This would allow engineers to design more efficient heat transfer systems that don’t waste as much energy. This concept might also prove to be a game-changer for many processes that aren’t as widely embraced because they’re so energy-prohibitive, like desalination.
Perhaps almost as importantly, the concept could help inform the process of liquid cooling, which is used to draw heat away from electronics in environments like data centres. Being able to halt bubble formation, as well as speed things up, could be just as critical to saving resources.
[MIT]