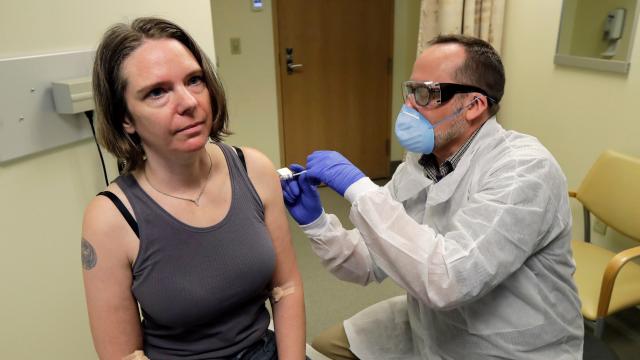
On Monday, the U.S. National Institutes of Health (NIH) announced they had started the first human trial of a possible vaccine for SARS-CoV-2, the coronavirus behind covid-19, with the first test subject receiving their shot that very day. But while this trial, based in Seattle, and other upcoming ones are certainly good news, it will still take lots of time and good luck for any vaccine to reach the public.
The experimental vaccine is known, for the time being, as mRNA-1273. It was created through a collaboration between the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and the biotech company Moderna based in Cambridge, Massachusetts. Though the NIH is funding the trial itself, additional funding for the manufacture of the vaccine was provided by Coalition for Epidemic Preparedness Innovations (CEPI), a foundation started in 2017 by the Bill and Melinda Gates Foundation and other partners specifically to fund vaccine research for emerging infectious diseases like covid-19.
This is a Phase 1 clinical trial, meaning that scientists are primarily interested in testing how safe mRNA-1273 is to use on healthy people and whether it comes with any dangerous side effects. Different amounts of the vaccine will be given to people to gauge the dose that’s most safely effective. While researchers will likely monitor things like the immune response to the coronavirus from volunteers, those results alone can’t tell us whether the vaccine will work. In total, the trial will involve 45 volunteers ages 18 to 45, recruited over a six-week span, who will take two doses one month apart, then be monitored for the next year.
“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said Anthony S. Fauci, director of the NIAID, in a statement released by the NIH. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
Some scientists have criticised how this record speed was accomplished, though. Moderna’s vaccine candidate hasn’t undergone the sort of animal trials that experimental drugs and vaccines typically go through. Experimental treatments aren’t legally mandated to undergo animal testing, but flouting these unspoken rules—even in times of crisis—could set a bad precedent going forward or even endanger people’s lives.
Previous attempts to create coronavirus vaccines for SARS have stumbled because they triggered an overreaction by the immune system in test animals, where exposure to the virus in the wild could actually make the vaccinated animals even sicker than usual. While newer vaccines should have theoretically solved this problem, animal trials would help rule out that possibility.
There are also still lingering questions about Moderna’s approach to its vaccine, which relies on something called messenger RNA, or mRNA. mRNA vaccines work by programming cells in the body to produce an antigen specific to the target germ; that antigen then primes the immune system to recognise and attack the germ. This is in contrast to the conventional vaccine, which relies on using at least some part of the pathogen (possibly modified) to train the immune system.
In theory, mRNA vaccines should have their advantages over older types of vaccines, with the hope being that they’ll be easier, cheaper, and quicker to mass-produce and could be adapted to a wide array of diseases.. But practically, no mRNA vaccines have been approved by any government yet, with most candidates still in early development. In other words, the technology behind mRNA vaccines is still largely untested.
[referenced url=” thumb=” title=” excerpt=”]
Even if Moderna’s vaccine doesn’t work, it’s not the only potential one set to be tested by the U.S. government and others. More than a half-dozen vaccine candidates are in development, including one by Peter Hotez, dean for the National School of Tropical Medicine at Baylor College of Medicine in Texas, and his team.
“I’m more optimistic about our vaccine because it uses an established technology that has led to the licensure of other vaccines, including hepatitis B and HPV,” Hotez told Gizmodo, referring to his team’s approach of modifying a key protein taken from the virus. A version of their vaccine has already been tested in animals successfully, though as a vaccine for SARS, not the closely related coronavirus behind covid-19.
Regardless of which vaccine proves most timely or effective, they won’t be of any immediate help. We may simply have to hunker down and endure covid-19 as best as we can until then.
“In any case, all of these vaccines need to be carefully evaluated for efficacy and safety and that could easily take one to two years, as stated by Dr Fauci,” Hotez said.