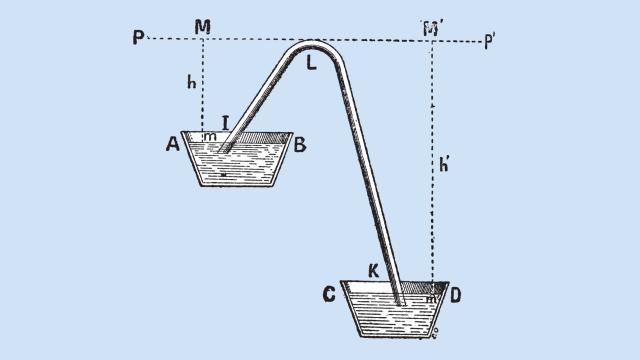
If you’ve ever siphoned petrol from a tank, you’ll know that the height between the source and the destination of the liquid is important. But scientists have shown that the height limit of a siphon is much bigger than we thought.
The maximum height of a siphon is usually thought to be defined by the atmospheric pressure of wherever you happen to be siphoning. In a paper published in Scientific Reports, researchers from the Queensland University of Technology explain why that’s the case:
This limit arises because the pressure in a siphon above the upper reservoir level is below the ambient pressure, and when the height of a siphon approaches 10 meters [10m], the pressure at the crown of the siphon falls below the vapour pressure of water causing water to boil breaking the column.
And when the column breaks, your siphon stops working. Typically, at sea level, that happens when the siphon reaches 10m in height. But what the Australian team realised was that the “boiling” they refer to — perhaps less confusingly referred to as cavitation, because it’s more the formation of bubbles because of lower pressure rather than anything to do with heating — is a result of excess gas in the water inside a siphon. As the pressure changes, dissolved gas forms into bubbles and then disaster strikes.
So they carried out a set of experiments using degassed water instead of the usual stuff that comes out of your tap. They left water under a vacuum for more than three weeks, forcing large quantities of gas out of it — then they tested out how that affected the ability to create a taller siphon.
Their results show that it’s possible to create a siphon that is 15m tall which operates quite happily at seal level. That’s because, according to the researchers, the degassing of the water prevents the cavitation from occurring: Without gas in the liquid, which can form bubbles as the pressure changes, the water’s essentially “stronger” and much harder to tear apart.
The researchers speculate that their small experiment suggests that the limit can be pushed further still — at least to 91m and maybe even beyond. “If tensions as high as the transient tension of several 100 bar can be maintained at the apex of a siphon,” they write, “then in principle a siphon should work up to a height of several kilometres. However, it would be challenging to verify this experimentally, requiring a helicopter or UAV.”
Sounds like a challenge.
Image by Morphart Creation/Shutterstock