We used to think that technology was about devices. We were wrong. Those feeble plastic and glass exoskeletons are nowhere near as important as the batteries that power them. Which is why the race to a better battery is fuelled by insane hype — threaded with genuine innovation.
Picture: University of Illinois
The market for a better battery is potentially enormous. Yet as our gadgets and cars have evolved, the batteries powering them have remained pretty much unchanged. And while the press is full of reports of eureka-moment “breakthroughs,” it’s turned out to be remarkably difficult to commercialize any of this new technology on a broader scale, as journalists like Kevin Bullis and Steve LeVine have chronicled (more on that later). Making battery magic in a lab is one thing. Figuring out how to reproduce that magic safely, in a factory, millions of times over, at a price that’s competitive? That’s another.
Yet the race continues: Electric car makers are looking for cheaper, lighter, more powerful and durable cells. Electronics makers are looking for more reliable cells that can charge faster and last longer. For makers of medical implants and even wearable technology, it’s a battery small enough to “disappear.” Meanwhile, renewable energy companies are looking for batteries that can charge and discharge thousands and thousands of times and remain stable.
The breakthroughs that we seem to hear about on a weekly basis are real. But there’s an increasingly apparent gap between a breakthrough and its adoption. I looked into three areas of buzz-y battery research to find out how close they are to — as that tired old adage goes — truly changing the world.
The Solid State
Let’s start with an emerging technology that does away with a very dangerous problem with current lithium ion batteries: Their enthusiasm for bursting into flame without warning. These are called solid state batteries — there are many types — and to understand how they avoid instantaneous conflagration, it helps to know a bit about why this phenomenon occurs in lithium ion batteries in the first place.
Most conventional lithium ion batteries are made of up two electrodes (the anode and cathode), separated by some sort of liquid electrolyte, or the medium that conducts the lithium-ions moving from anode to cathode. The problem is that this electrolyte is very flammable — if it’s damaged or punctured, the battery will catch fire. Leading to things like, uh, this:
Solid state batteries do away with the liquid electrolyte altogether. Instead, they use a layer of some other material, usually a mixture of metals, to conduct ions between the electrodes and create energy.
But that’s only half the reason solid state technology is so exciting. Because there’s no liquid component in these cells — and because they require fewer extra layers of insulation and other safeguards — they tend to be smaller, lighter, and more adaptable than their fire-happy predecessors. That makes them very interesting to carmakers looking for a lighter, safer battery for their electric vehicles. The Department of Energy’s Advanced Research Projects Agency-Energy, or ARPA-E, is running multiple projects to either develop solid state lithium ion batteries, or solid state batteries that do away with lithium altogether.
Then there’s a leader in solid state, Sakti3, an 8-year-old company based in Ann Arbor headed up by CEO Ann Marie Sastry. A profile from MIT Technology Review’s Kevin Bullis gives us a glimpse into the work Sakti3 and Sastry are doing, which focuses on figuring out how to build solid state lithium ion batteries at scale:
She is also developing manufacturing techniques that lend themselves to mass production. “If your overall objective is to change the way people drive, your criteria can no longer only be the best energy density ever achieved or the greatest number of cycles,” she says. “The ultimate criterion is affordability, in a product that has the necessary performance.”
Sakti3’s work sounds exciting, but the company has been extremely secretive about its technology, so we don’t know exactly what it uses as its electrolyte — which could certainly end up affecting the cost or manufacturability of these batteries on a larger scale. We do know Sakti3 has attracted investments from major players, including GM’s venture arm, and claimed last year that it had doubled the energy density of the average lithium ion battery. Another solid state company, QuantumScape, is similarly quiet — but is rumoured to be working on similar ideas with solid state tech.
So, why aren’t we riding around with solid state batteries under our hoods? It’s still fairly early days for commercialising on that scale. One of the biggest challenges with battery tech isn’t just the electrochemical secret sauce, it’s replicating that secret sauce in a factory, for a price lower than that of conventional cells, with greater regularity, at massive scale.
It’s a paradigm that the author Steve LeVine knows well. LeVine’s new book The Powerhouse, published this spring, is a deep dive into the rise — and fall — of a company attempting to commercialize just one of those Eureka-Game-changing-Aha-Moment-Battery-Innovations. He spent years following Envia, a battery startup that eventually secured a contract with GM to supply its cathodes, made from nickel, manganese, and cobalt, to power GM’s Volt. Until it all fell apart when the cathodes didn’t perform the way Envia claimed they would.
As LeVine explained to me on a recent call — and as he echoed in a story in Quartz this week, the most exciting thing in battery tech right now isn’t the battery. It’s the manufacturing process. “I’ve gotten very excited about what’s possible by figuring out how to bring down costs through manufacturing breakthroughs,” he said, pointing out that the Department of Energy is now focusing on staging competitions that ask entrants to focus on innovating the manufacturing process rather than the electrochemical science of the batteries themselves. “I think that’s the place to watch,” he added.

The Tesla Gigafactory under construction in March, via the Tesla Forum.
Even Elon Musk is trying to solve this particular problem. His Gigafactory, which is currently underway in Nevada, is a massive bet on the idea that Tesla can beat out its competitors simply by putting the entire battery manufacturing process under one roof. Keep in mind, this is for batteries that aren’t particularly groundbreaking. But this game is about economies of scale — and even Musk is enduring criticism that his battery factory might be obsolete before it opens as other breakthroughs in battery tech emerge. That’s a big and polemical theoretical, but it helps illustrate how mercurial the battery industry is right now.
The Aluminium Air
Even though lithium is the king of battery materials, it has plenty of other drawbacks besides bursting into flames. Not only is it expensive to mine, but it’s less efficient than some other materials at releasing electrons, as Chemistry World recently explained, which makes it slower to charge and discharge.
So, what about batteries that don’t need any lithium at all, some of which could charge your phone in seconds — at least theoretically? An Israeli company named Phinergy has talked up one exciting but fraught contender over the past few years: An aluminium air battery. In these batteries, one electrode is an aluminium plate. The other is oxygen. More specifically, oxygen and a water electrolyte. When the oxygen interacts with the plate, it produces energy.
Aluminium air batteries have been around for a long time, though interest in them has intensified over the last few years. A much-cited 2002 study from the Journal of Power Sources brought it into the spotlight, when a group of researchers argued that aluminium-air batteries are the only feasible replacement for gasoline. In theory, these batteries could have 40 times the capacity of lithium ion batteries, and Phinergy says they could extend the range of EVs to 1,000 miles.
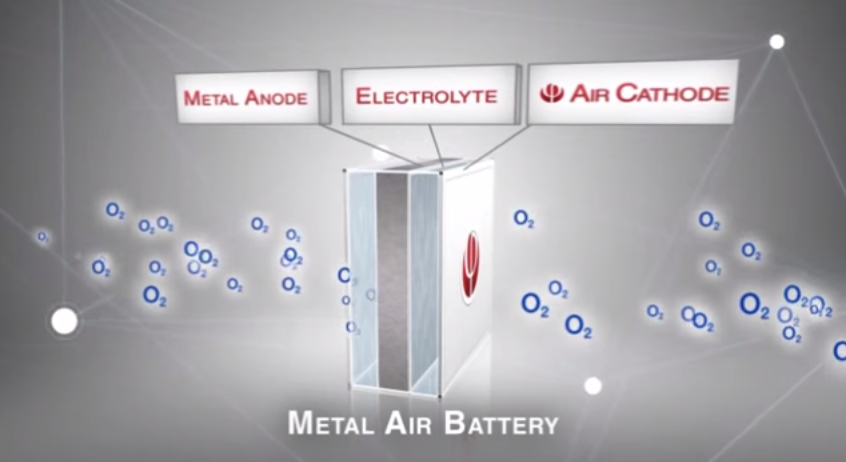
Image: Phinergy.
So, it’s time to ask again: Why aren’t we all driving around in oxygen-powered cars? Well, the chemical reaction that produces energy in these batteries also happens to come with a considerable drawback. As it interacts with the oxygen, the aluminium degrades over time. It’s a type of battery called a “primary” cell, which means current only flows one way, from the anode to the cathode. That means they can’t be recharged. Instead, the batteries have to be swapped out and recycled after running down.
That’s a big infrastructure problem when it comes to widespread use. “For EVs that might be an ok situation once the infrastructure is in place for service stations to swap out new and used batteries from vehicles,” explained University of Michigan Battery Lab’s Greg Less via email. “But until that occurs, a secondary [rechargeable] cell, like Lithium-Ion will be preferable.” Aluminium air batteries certainly wouldn’t be feasible for gadgets, because they would need to have their batteries swapped out regularly.
Still, research is continuing on aluminium air, and there are several companies claiming they will bring it to market within the next few years, including Phinergy. A company called Fuji Pigment also claimed recently that it had made a huge leap forward. Fuji says that it’s figured out a way to protect the aluminium with insulating materials, so it would be able to recharge without being swapped.
Image: Stanford.
Even if the aluminium air contenders fail, researchers are increasingly pointing towards aluminium as the battery material of the future. It’s a hot field right now: Just while I was writing this article, another piece of battery news was announced — this one from a lab at Stanford that uses aluminium and graphite as electrodes, connected by a safe liquid electrolyte. The group at Stanford says their battery can charge a smartphone in under a minute and can be “drilled through” and still remain functional. Of course, more research remains to be done.
The Microbattery
Another major issue with conventional batteries is their size. While almost every other part of our electronics get smaller, batteries are still pretty hefty. For example, the newest Apple laptop is defined by its battery size — which, even though it’s designed in a super-efficient tiered structure, still takes up most of the space in the body.
This is a problem that goes way beyond laptops, though. Think of medical implants, which need a power supply small enough to sit inside the human body. Or ambitious long-term airborne craft projects like Solar Impulse, which need feather-light batteries to store energy. Finally, what about Project Jacquard, which seeks to wire computers into our very clothing — hopefully without a pound of lithium tucked into a pocket.
More and more research is focusing on what are called “3D” microbatteries. What’s the difference between 2D and 3D? Well, think of a 2D version as a simple sheet cake: There are two electrodes, separated by an electrolyte. These can get super-thin, but you’re limited to a very thin cake with a pretty low power output.
In comparison, a 3D battery is more like a roll cake (OK, it’s an imperfect metaphor) where you can increase the surface area of the electrodes by tightly interlocking them in microscopic layers. By increasing the surface area, you make it easier for ions to travel from one electrode to the other — which increases the battery’s power density, or the rate at which it charges and discharges.
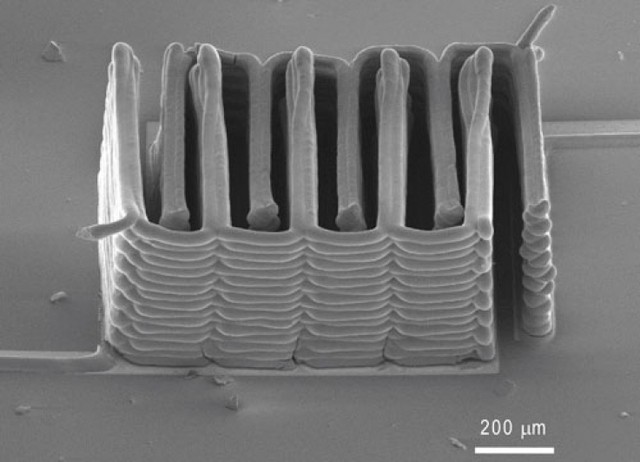
Image: Harvard.
Scientists are exploring many ways to manufacture these tiny wonders. In 2013, a team from Harvard used a 3D printer to get the extreme precision needed to intertwine nano-sized anodes and cathodes using a lithium “ink.”
But more recently, a team from University of Illinois published a paper showing how they used a technique called holographic lithography to make a 3D battery. In it, super-precise optical beams are used to create a 3D structure — in this case, the electrodes — out of a photoresist (think of it as a three-dimensional unexposed negative) which in turn become the battery itself. Why is this better than 3D printing? Well, for one thing, holographic lithography isn’t as nascent as 3D printing, so it may have more promise when it comes to scaling up.
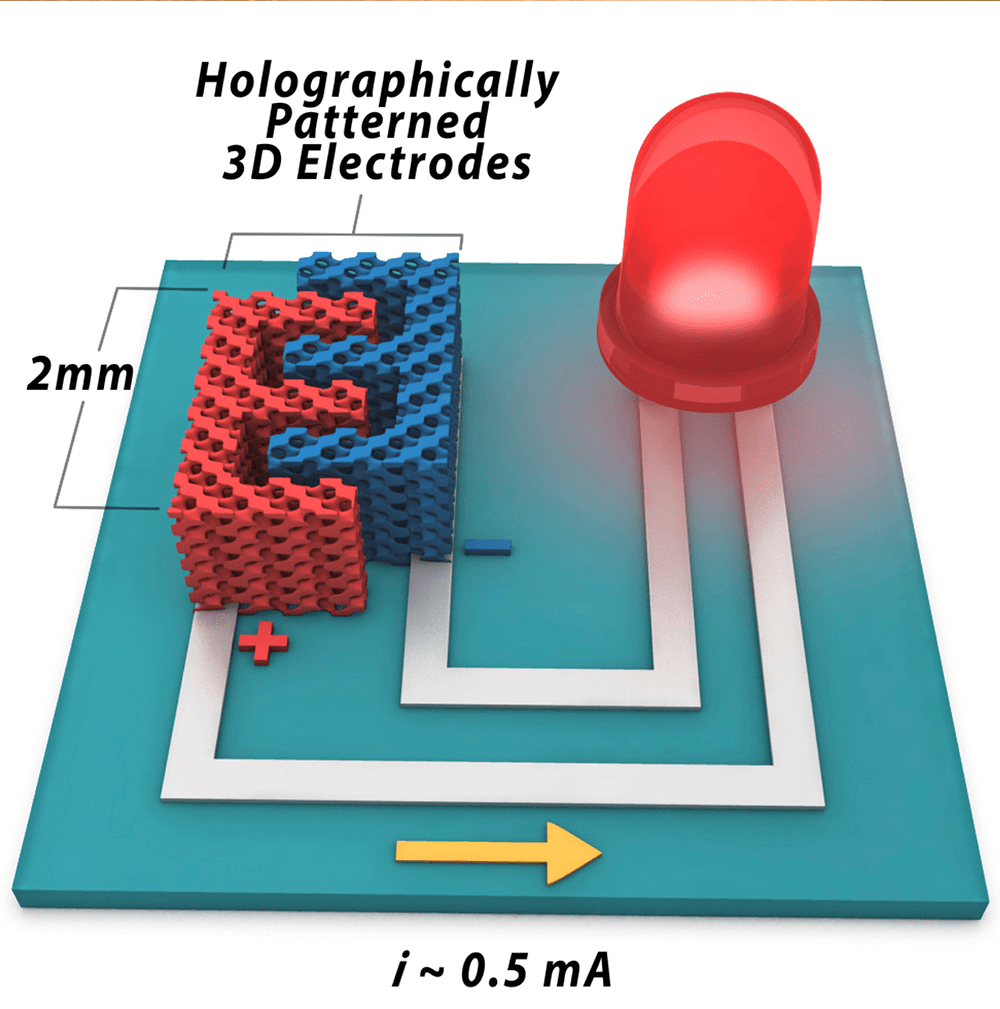
However, like all batteries, there’s a tradeoff here between power density, the rate that a battery produces energy, and energy density, the overall capacity of a battery — as GizMag’s Brian Dodson explained in a post about the research. It’s tough to be good at both of those things, but that’s exactly what the Illinois team is trying to do. If they succeed at commercialising their tech, it could be big. Again, that’s a mighty “if.”
Indeed, one of the paper’s authors, UI professor William King, told Gizmodo via email that the big hurdle now is figuring out how to turn this into a commercial technology. “Since our first article was published on this technology, we’ve managed to increase the battery energy density by about a factor of 3, by using new, higher energy materials,” he said. Still, “the key challenge is manufacturing scale-up, which we have been working on diligently.”
What’s Going on Inside?
One of the problems with replicating a breakthrough in a lab is that often, we don’t really know what’s happening inside the battery itself. This sounds simple, but it’s a massive challenge and arguably the biggest thing holding up battery innovation: We can’t actually observe what’s going on at a molecular level. It’s why so many battery breakthroughs seem to be accidental or unexplainable — and why they fall flat when their inventors can’t reproduce the same effects in a controlled way.
So I talked with one researcher who isn’t focusing on building batteries — he’s focusing on seeing inside of them. Michael Toney, of the SLAC National Accelerator Laboratory, is leading the way towards actually observing what’s happening inside a battery without cracking it open or disturbing the process.
Toney and his colleagues are using spectroscopic imaging and nanoscale x-rays to understand exactly what’s happening inside, say, a lithium ion battery when it’s charging. As Toney told me, the ultimate goal is to be able to view what’s happening on an atomic level. For now though, his team can view the chemical processes to determine how, for example, an anode might be leading to voltage fade, or a gradual loss of energy over time.
Eventually, Toney says the same technology could lead to software that can realistically tell you how your battery is doing — not just guess, as your phone’s little bar system does now. But that’s small potatoes compared to being able to see how batteries actually work. Because the strangest thing about the race to build a battery than can replace fossil fuels isn’t just that there are so many contenders — it’s that knowing why they succeed or fail is so incredibly hard.
While we want a breakthrough battery to be as simple as a successful experiment, it increasingly seems like finding it will be a long, incremental research effort that will see many successes and failures before all is said and done. After all, this is the Infrastructure Age. Don’t expect it to end before it even begins.