It’s believed that last week the Syrian government murdered hundreds of its own civilians with chemical weapons. We don’t know which weapon they used, but we do know it’s one of a handful of chemicals called nerve agents.
Nerve agents were invented accidentally by a Nazi-era scientist trying to make a more effective pesticide. That’s why these molecules are so similar in their structure to the most common class of insecticides, chemicals called organophosphates because they contain carbon-based (“organic”) groups coupled to a phosphorous atom. And the way a nerve agent kills you is very similar to the way an organophosphate pesticide kills an insect.
It looks like last week’s massacre could lead to war (the White House is talking about a missile strike and European powers are talking about military intervention). With that in mind, here’s a look at the science of what happened.
This post originally appeared on Puff the Mutant Dragon, and has been republished with permission.
How do we know the Syrian govt used nerve agents?
The symptoms are unmistakable. For example, the pupils of victims’ eyes had narrowed to the size of pinpoints. That’s exactly what happened to the German scientist who invented tabun, the first nerve agent. The pinpoint pupils is a hallmark of nerve agent poisoning. There is no other chemical weapon that would cause these kinds of symptoms.
What did they use?
All the nerve agents cause similar symptoms, so there’s no way to know for sure without a sample for testing.
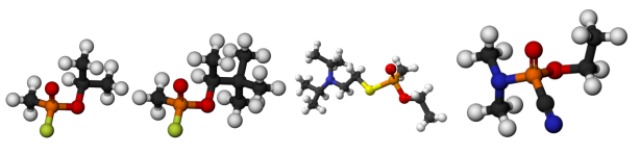
From left to right: Sarin, soman, VX and tabun. The orange atom is a phosphorus; that’s why these are all “organophosphates”. Carbon is black, nitrogen is blue, oxygen is red, sulfur is yellow and fluorine is green. The phosphorus atom is the part of the molecule that reacts with acetylcholinesterase.
Reuters says the weapon was probably sarin because the Syrian govt apparently has stockpiles of that particular chemical. Sarin is a chemical with an ugly history, by the way; it seems that back in the 1950s the British government illegally tested sarin on some unsuspecting British soldiers who were told they were participating in a test for a new cold medicine. One of these soldiers died in the process. The Guardian has the story on that at this link.
These four chemicals are more challenging to make than something, like, say, crystal meth. They’re not something you could cook in your backyard. But any halfway decent synthetic organic chemist could make them. So pretty much any government that wants them badly enough can get them…which is why they’re a problem.
What happens to you when you’re poisoned by a nerve agent?
Your brain loses the ability to control your muscles, including the muscles in your diaphragm that enable you to breathe, so you go into convulsions (your muscles contracting uncontrollably), drool, lose control of your bladder, your chest tightens up, you stop breathing and eventually die from respiratory failure. It’s not an easy way to die. Whoever decided to fire this at civilians is one sick puppy, no two ways about it.
How do nerve agents work?
Your brain sends signals to your muscles through nerves called motor neurons. At the point where the motor neuron meets the muscle, the neuron pumps out a chemical called acetylcholine that tells the muscle to contract. Acetylcholine gets broken down by an enzyme called acetylcholinesterase (I’ll call it A-prime for short). It’s an esterase because it breaks an ester bond in acetylcholine; an ester is two oxygens bonded to a carbon in the middle of a carbon chain. Ester bonds tend to break down very slowly in water, but an enzyme like A-prime accelerates this reaction so it happens quickly.
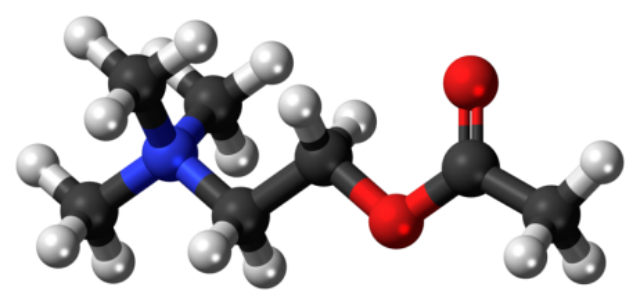
This is acetylcholine. It triggers your muscles to contract. Acetylcholinesterase (A-prime) breaks it up rapidly so that your muscles don’t stay contracted once the signal from your brain telling them to contract is over.
An organophosphate like sarin slides into a slot on the A-prime protein like a key fitting into a lock — because its shape enables it to slide right in there. Once it’s in the slot, it reacts with an amino acid called serine in the A-prime molecule, and now it’s stuck onto A-prime for good. A molecule of A-prime that has an organophosphate stuck to it can no longer break down acetylcholine, so acetylcholine hangs around and your muscles think your brain is telling them to stay permanently contracted because the acetylcholine doesn’t go away — it’s still there. So your muscles contract and stay contracted — and at that point you can no longer breathe. That’s why the pupils of your eyes shrink to pinpoints (because the band of muscle called the iris has contracted and can’t relax).
The reaction between sarin and A-prime is very fast and very irreversible. That’s why sarin (and other nerve agents) are deadly even at very low doses — because even at low concentrations nerve agents react with A-prime very quickly. And you can absorb nerve agents through your skin. These compounds are actually liquids at room temperature, by the way; a lot of people call them “nerve gas”, and true, they are fairly volatile, meaning they can evaporate pretty easily. But if you spray them you scatter a mist of tiny droplets called an aerosol, just like the spray from a can of air freshener, and people can inhale these tiny droplets very easily.
Is there an antidote?
Yes. There’s a chemical called 2-PAM which looks like this:
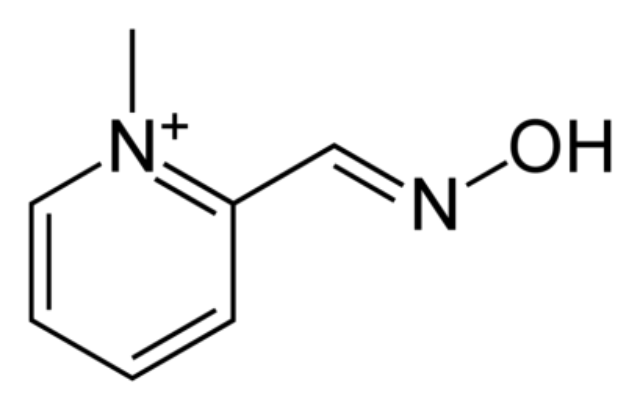
On one end of 2-PAM is what’s called an oxime group, a nitrogen double-bonded to carbon with an OH on it. This group is very polar (i.e. electrons aren’t shared equally) and the OH is electron-rich, so it can react with the phosphorus from sarin that’s stuck to the serine in A-prime. When it does that, A-prime is freed up again to get back to doing its job.
This doesn’t always work because an organophosphate that’s already reacted with A-prime can actually undergo a subsequent reaction called “ageing”** which ensures it cannot be removed by 2-PAM. This “ageing” reaction is slow for most nerve agents, however.
Typically doctors co-administer 2-PAM together with atropine, a chemical from the Jimson weed plant that blocks acetylcholine from binding to a specific type of receptor on muscle cells, so it helps you recover from nerve agent poisoning.
Depending on the agent and how much you inhaled, injecting you with some 2-PAM and atropine can save your life — IF the doctor can get to you quickly enough. I have a feeling the Syrian army wasn’t exactly making way for the ambulance though.
How were these invented?
By accident. A German chemist was trying to come up with a powerful new pesticide. He didn’t know any of this stuff about acetylcholinesterase; he just knew that some phosphorus compounds with carbon-based groups coupled to them seemed to do a great job killing bugs. He didn’t really know why, but he knew he was onto something. So he kept on tweaking these compounds, making little changes, adding new and different chemical groups then testing them to see whether they were even better at killing insects, and one day he came up with something that smelled vaguely like apples. He took a whiff and nearly killed himself.
Rumours about this new compound got round to the German government. A chemical that’s lethal in tiny doses and it smells like fruit? You better believe Hitler was interested. Thatcrazy bastard was always looking for new ways to kill people.
Why don’t pesticides like malathion kill humans too if their structure is so similar to the nerve agents?
Take a look at malathion below:
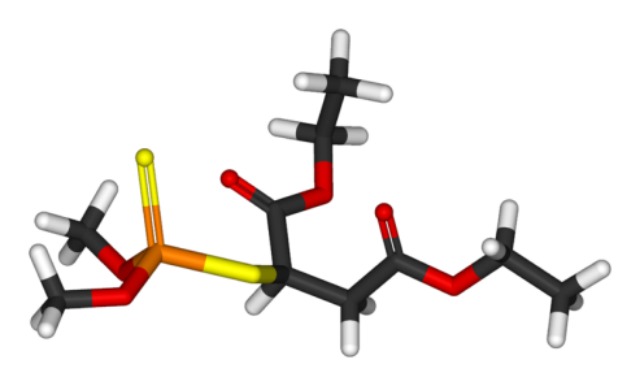
You can see it looks like one of the nerve agents but it’s different enough you can tell them apart, right? And these differences mean that the organophosphate pesticides are typically more toxic to insects than they are to humans, because the structural differenecs change the way the chemical is absorbed and metabolized and so forth. Malathion, for example, has reasonably low toxicity to humans because your body breaks it down and excretes it pretty rapidly*. Like most organophosphate pesticides, it also breaks down fairly rapidly in the environment.
And yet, many of the organophosphate pesticides are still highly toxic to humans. Parathion, for example, is so toxic you could call it a weak nerve agent. Farm workers are the people most at risk, and chemicals like parathion have in fact caused numerous accidental deaths among farm workers, especially in developing nations. Many of the most toxic chemicals used in agriculture are organophosphate pesticides.
It’s remarkable how much history organophosphate pesticides and chemical weapons share in common. VX, for example, is based on a nerve agent called VG, which was originally made and sold as a pesticide until somebody finally noticed it was insanely toxic to humans.
Hmm. I don’t really like the sound of these organophosphate insecticides. Aren’t there alternative chemicals that kill insects?
Alternatives include the carbamate pesticides, the organochlorine pesticides and the pyrethroids.
The carbamates work by knocking out insect acetylcholinesterase. The way they disable acetylcholinesterase is a little different from the way an organophosphate works. Nonetheless, some of them are also very dangerous to human or animal health — carborufan comes to mind. Most of the carbamates tend to break down quickly in the environment.
The organochlorine pesticides are hydrocarbons with chlorine atoms attached. DDT is the most notorious. Most of the organochlorine pesticides tend to break down slowly in the environment. Since they’re so fat-soluble your body and those of other animals can only get rid of them slowly, which is why they tend to accumulate going up the food chain. The EPA has banned or restricted most of these because of their environmental impact.
The pyrethroids are compounds based on an insect-killing chemical found in the chrysanthemum flower. They work in a completely different way from the organphosphate/carbamate families; their targets are ion channels in nerve cells. Your liver can break most of these down very rapidly, and they tend to be much less toxic to birds and mammals than most organophosphate pesticides so they’ve been becoming more and more popular, especially for indoor uses. Most of them tend to break down quickly in the environment. That doesn’t mean they’re totally benign, however. They kill beneficial insects like bees, and they’re extremely toxic to fish.
There are other insecticides out there, but these are the biggest classes.
Well, ok. I can see not all pesticides are created equal. But it sounds like even the most benign of them come with drawbacks. Isn’t there another way to kill insects besides using these compounds?
Glad you asked. There is, although a lot of people don’t like it. I’ll return to that in a future post. For now let’s get back to Syria.
What should we do about it?
Sure, I have my opinions about what we should and should not do just like anyone else. But this isn’t a political blog, and my two cents about politics isn’t worth all that much either way. So I’ll leave that part up for debate.
*The important point here is that malathion is really a “prodrug” if you will; inside the the human liver or an insect, cytochrome P450 enzymes convert it to malaoxon, i.e. that double-bonded sulfur is replaced by an oxygen, and malaoxon is the compound that actually binds and inactivates acetylcholinesterase. In humans and other mammals, carboxylesterase enzymes typically break malathion down rapidly which limits the amount of malaoxon that gets produced. Some insects seem to have evolved resistance to malathion through mutations that result in high levels of carboxylesterase enzymes so they can tolerate much higher levels of the pesticide.
**The actual reaction is hydrolysis of one of the ester bonds to the phosphorus atom attached to serine.
Puff the Mutant Dragon is an analytical chemist in the biotech industry with a background in biochemistry who maintains a little blog to try and spread the joy of science, and combat misunderstanding. You can read more of Puff’s work over at his/her blog.
